G Protein Coupled Receptors
Sakshi Education
Cells respond to various signals in order to execute cellular functions. Signals can be physical or chemical or mechanical in nature. Some signals like light are physical in nature and some like touch are mechanical in nature and some like hormones are chemical in nature. Majority of the signals are of chemical nature like nutrients, growth factors, hormones, neurotransmitters, and extracellular matrix components. These substances can exert their effects locally, or they might travel over long distances. For instance, neurotransmitters are a class of short-range signaling molecules that travel across the tiny spaces between adjacent neurons or between neurons and muscle cells. Other signaling molecules must move much farther to reach their targets. One example is follicle-stimulating hormone, which travels from the mammalian brain to the ovary, where it triggers egg release.
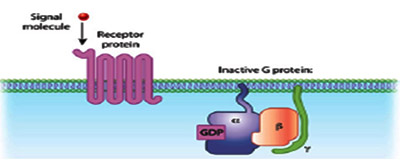
Cells have transmembrane proteins which receive the signaling molecules outside the cell and are known as receptors. Receptors after binding to signaling molecules transmit the signal through a sequence of molecular switches to initiate internal signaling pathways and mount a physiological response. Although the cells possess hundreds of receptor types and varying cell types have different populations of receptors, binding of a signaling molecule to a receptor is very specific. Receptors can also respond directly to signals like light or pressure, which makes cells sensitive to events in the atmosphere. Signaling molecules are often are too large or too charged to be able to cross the plasma membrane, therefore receptors are generally found on the plasma membrane. They help the signaling molecules to execute their functions without actually entering the cell. Some molecules like gases eg. Nitrous oxide and steroid hormones eg.estrogen can easily pass through the plasma membrane. Receptors for such molecules are located internally in the cell mostly on the nuclear membrane.
Based on this, cell signaling pathways are divided into two, those involving intracellular receptors and cell surface receptors. Cell surface receptor based signaling is further subdivided based on effector mechanism used for signaling. Receptors possessing intrinsic effector activity are grouped into one category and receptors lacking intrinsic activity are grouped into another. Tyrosine kinases and ligand gated ion channels belong to the first category and G protein coupled receptors belong to the second category.
G-protein-coupled receptors (GPCRs) are the largest and most diverse group of membrane receptors in eukaryotes. This class lacks intrinsic effector activity. Signals in the form of light energy (photons of light),chemical odorants, divalent cations, monoamines, amino acid, nucleoside neurotransmitters, peptides, lipids, sugars, and proteins are received by the GPCRs. GPCRs are found in many eukaryotes including animals, plants, fungi, and protozoa. About a 1,000 different GPCRs specific to different signals are found in humans alone. GPCRs consist of a single polypeptide that is folded into a globular shape and embedded in a cell's plasma membrane. Seven segments of this molecule span the entire width of the membrane with an extracellular amino terminus and are hence called seven-transmembrane receptors and the intervening portions loop both inside and outside the cell. The extracellular loops form part of the pockets at which signaling molecules bind to the GPCR. The cytosolic domains are coupled to GDP. In humans there are about 800 7TM proteins, 150 of them are coupled to G proteins. In plants there exists a simpler version where only one 7TM is known to be associated with G proteins.
Based on the differences in the sequences especially in the transmemnrane helices, The GPCT superfamily is divided into subfamilies. Family 1 is the largest which includes opsins, odorant receptors, monoamine receptors, chemokine receptors, purine receptors, opiate receptors, receptors for large glycoprotein hormones, Thyroid Stimulating Hormones, Follicle Stimulating Hormones, and Luteinizing Hormone. Family2 exhibits a large variation in sequence to the family 1 and includes receptors for peptide hormones like parathyroid hormone, parathyroid hormone related protein and calcitonin. Family 3 include metabotropic glutamate receptors, extracellular Ca2+ sensing receptorsand putative taste and pheromone receptors.
G proteins are specialized, small guanosine triphosphate (GTP) and guanosine diphosphate (GDP) binding proteins. Some G proteins are monomeric while others are multimeric. Ras and Rho are classical examples of monomeric GTPases. The G proteins that associate with GPCRs are heterotrimeric, containing three different subunits: an alpha subunit, a beta subunit, and a gamma subunit. Two of these subunits — alpha and gamma are attached to the plasma membrane by lipid anchors. G protein alpha subunit binds either GTP or GDP depending on whether the protein is active (GTP) or inactive (GDP).
In the absence of a signal, GDP attaches to the alpha subunit, which ineracts with beta subunit of beta-gamma dimer and the entire G protein-GDP complex binds to a nearby GPCR. When a signaling molecule binds with the GPCR, a change in the conformation of the GPCR occurs resulting in activation of the G protein, and GTP physically replaces the GDP bound to the alpha subunit. This exchange process occurs due to the intrinsic Guanine Exchange Factor (GEF) activity of the GPCR. Monomeric G proteins require the aid of a extrinsic GEF that replaces the GDP with GTP in response to upstream activation. Consequently, the G protein subunits dissociate into two parts: the GTP-bound alpha subunit and a beta-gamma dimer and become available to interact with downstream effectors.. Both parts still remain attached to the in spite of not being attached to the GPCR.
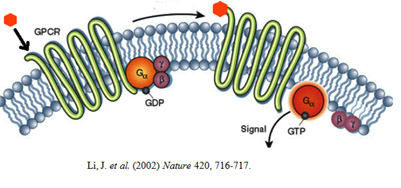
Li, J. et al. (2002) Nature 420, 716-717.
Anchoring on to the membrane allows them to diffuse laterally to interact with other membrane proteins. G proteins remain active as long as their alpha subunits are joined with GTP. However, when this GTP is hydrolyzed back to GDP, the subunits once again assume the form of an inactive heterotrimer, and the entire G protein reassociates with the now inactive GPCR. GTPase activating proteins (GAP) facilitate the GTP hydrolysis leading to an accelerated termination of the signaling. Thus G proteins act as Molecular switches that are either turned on/off by the signal-receptor interactions on the surface of plasma membrane. Whenever a G protein is active, both its GTP-bound alpha subunit and its beta-gamma dimer can relay messages in the cell by interacting with other membrane proteins involved in signal transduction. Specific targets for activated G proteins include various enzymes like phospholipase C and phospholipase D that produce second messengers, as well as certain ion channels like K+ and Ca2+ channels, that allow ions to act as second messengers. Some G proteins stimulate the activity of these targets, whereas others are inhibitory.
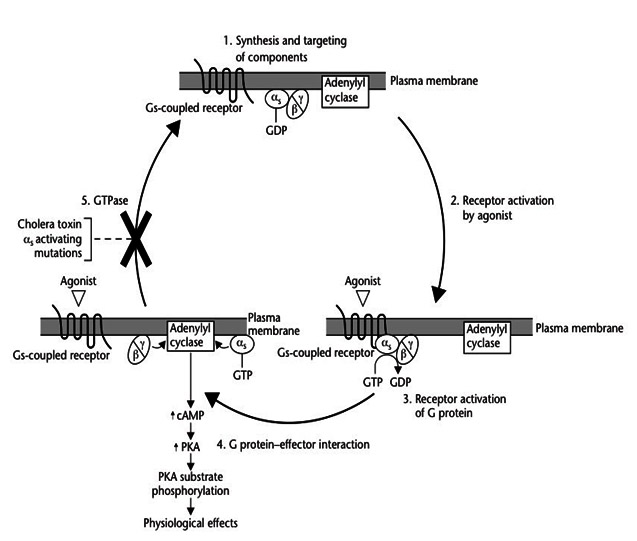
The process of signal perception depends on the local assembly of receptor protein complexes which exhibit slow diffusion kinetics. Therefore cells utilize small, freely diffusible molecules, second messengers for a faster signal, propagation. Activation of a single G protein can affect the production of hundreds or even thousands of second messenger molecules. Second messengers such as Ca2+, ROS, Nitricoxide, cyclic AMP [cAMP], diacylglycerol [DAG], and inositol 1, 4, 5-triphosphate [IP3] — are small molecules that initiate and coordinate intracellular signaling pathways. One especially common target of activated G proteins is adenylyl cyclase, a membrane-associated enzyme that, when activated by the GTP-bound alpha subunit, catalyzes synthesis of the second messenger cAMP from molecules of ATP. In humans, cAMP is involved in responses to sensory input, hormones, and nerve transmission, among others.
Phospholipase C is another common target of activated G proteins. Phospholipase C is a phosphodiesterase that hydrolyzes the membrane lipid phosphotidyl inositol to generate not one, but two second messengers — DAG and IP3. This particular pathway is critical to a wide variety of human bodily processes. For instance, thrombin receptors in platelets use this pathway to promote blood clotting. The seven-transmembrane (7TM) protein receptor in the plasma membrane activates a pathway that involves G proteins as well as cAMP-related pathways that modulate cellular signaling. Activated G alpha proteins inhibit (-) adenylyl cyclase, the enzyme that induces formation of cAMP, which in turn results in the activation of protein kinase A (PKA). This in turn activates a molecule called cAMP-responsive element-binding protein (CREB), which modulates gene transcription. GO proteins can also have a variety of other effects which include mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways. Activation of the enzyme phospholipase A2 (PLA2) may also occur, which induces the release of arachidonic acid (AA), as well as inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ levels. Subsequent activation of the MAPK and PI3K pathways results in the phosphorylation of extracellular signal-regulated kinases (ERKs) and protein kinase B (PKB), respectively. Activated PKB will subsequently phosphorylate and thereby inhibit the action of glycogen synthase kinase 3beta (GSK3beta), a major kinase in the brain.
References:
Cells have transmembrane proteins which receive the signaling molecules outside the cell and are known as receptors. Receptors after binding to signaling molecules transmit the signal through a sequence of molecular switches to initiate internal signaling pathways and mount a physiological response. Although the cells possess hundreds of receptor types and varying cell types have different populations of receptors, binding of a signaling molecule to a receptor is very specific. Receptors can also respond directly to signals like light or pressure, which makes cells sensitive to events in the atmosphere. Signaling molecules are often are too large or too charged to be able to cross the plasma membrane, therefore receptors are generally found on the plasma membrane. They help the signaling molecules to execute their functions without actually entering the cell. Some molecules like gases eg. Nitrous oxide and steroid hormones eg.estrogen can easily pass through the plasma membrane. Receptors for such molecules are located internally in the cell mostly on the nuclear membrane.
Based on this, cell signaling pathways are divided into two, those involving intracellular receptors and cell surface receptors. Cell surface receptor based signaling is further subdivided based on effector mechanism used for signaling. Receptors possessing intrinsic effector activity are grouped into one category and receptors lacking intrinsic activity are grouped into another. Tyrosine kinases and ligand gated ion channels belong to the first category and G protein coupled receptors belong to the second category.
G-protein-coupled receptors (GPCRs) are the largest and most diverse group of membrane receptors in eukaryotes. This class lacks intrinsic effector activity. Signals in the form of light energy (photons of light),chemical odorants, divalent cations, monoamines, amino acid, nucleoside neurotransmitters, peptides, lipids, sugars, and proteins are received by the GPCRs. GPCRs are found in many eukaryotes including animals, plants, fungi, and protozoa. About a 1,000 different GPCRs specific to different signals are found in humans alone. GPCRs consist of a single polypeptide that is folded into a globular shape and embedded in a cell's plasma membrane. Seven segments of this molecule span the entire width of the membrane with an extracellular amino terminus and are hence called seven-transmembrane receptors and the intervening portions loop both inside and outside the cell. The extracellular loops form part of the pockets at which signaling molecules bind to the GPCR. The cytosolic domains are coupled to GDP. In humans there are about 800 7TM proteins, 150 of them are coupled to G proteins. In plants there exists a simpler version where only one 7TM is known to be associated with G proteins.
Based on the differences in the sequences especially in the transmemnrane helices, The GPCT superfamily is divided into subfamilies. Family 1 is the largest which includes opsins, odorant receptors, monoamine receptors, chemokine receptors, purine receptors, opiate receptors, receptors for large glycoprotein hormones, Thyroid Stimulating Hormones, Follicle Stimulating Hormones, and Luteinizing Hormone. Family2 exhibits a large variation in sequence to the family 1 and includes receptors for peptide hormones like parathyroid hormone, parathyroid hormone related protein and calcitonin. Family 3 include metabotropic glutamate receptors, extracellular Ca2+ sensing receptorsand putative taste and pheromone receptors.
G proteins are specialized, small guanosine triphosphate (GTP) and guanosine diphosphate (GDP) binding proteins. Some G proteins are monomeric while others are multimeric. Ras and Rho are classical examples of monomeric GTPases. The G proteins that associate with GPCRs are heterotrimeric, containing three different subunits: an alpha subunit, a beta subunit, and a gamma subunit. Two of these subunits — alpha and gamma are attached to the plasma membrane by lipid anchors. G protein alpha subunit binds either GTP or GDP depending on whether the protein is active (GTP) or inactive (GDP).
In the absence of a signal, GDP attaches to the alpha subunit, which ineracts with beta subunit of beta-gamma dimer and the entire G protein-GDP complex binds to a nearby GPCR. When a signaling molecule binds with the GPCR, a change in the conformation of the GPCR occurs resulting in activation of the G protein, and GTP physically replaces the GDP bound to the alpha subunit. This exchange process occurs due to the intrinsic Guanine Exchange Factor (GEF) activity of the GPCR. Monomeric G proteins require the aid of a extrinsic GEF that replaces the GDP with GTP in response to upstream activation. Consequently, the G protein subunits dissociate into two parts: the GTP-bound alpha subunit and a beta-gamma dimer and become available to interact with downstream effectors.. Both parts still remain attached to the in spite of not being attached to the GPCR.

Li, J. et al. (2002) Nature 420, 716-717.
Anchoring on to the membrane allows them to diffuse laterally to interact with other membrane proteins. G proteins remain active as long as their alpha subunits are joined with GTP. However, when this GTP is hydrolyzed back to GDP, the subunits once again assume the form of an inactive heterotrimer, and the entire G protein reassociates with the now inactive GPCR. GTPase activating proteins (GAP) facilitate the GTP hydrolysis leading to an accelerated termination of the signaling. Thus G proteins act as Molecular switches that are either turned on/off by the signal-receptor interactions on the surface of plasma membrane. Whenever a G protein is active, both its GTP-bound alpha subunit and its beta-gamma dimer can relay messages in the cell by interacting with other membrane proteins involved in signal transduction. Specific targets for activated G proteins include various enzymes like phospholipase C and phospholipase D that produce second messengers, as well as certain ion channels like K+ and Ca2+ channels, that allow ions to act as second messengers. Some G proteins stimulate the activity of these targets, whereas others are inhibitory.
The process of signal perception depends on the local assembly of receptor protein complexes which exhibit slow diffusion kinetics. Therefore cells utilize small, freely diffusible molecules, second messengers for a faster signal, propagation. Activation of a single G protein can affect the production of hundreds or even thousands of second messenger molecules. Second messengers such as Ca2+, ROS, Nitricoxide, cyclic AMP [cAMP], diacylglycerol [DAG], and inositol 1, 4, 5-triphosphate [IP3] — are small molecules that initiate and coordinate intracellular signaling pathways. One especially common target of activated G proteins is adenylyl cyclase, a membrane-associated enzyme that, when activated by the GTP-bound alpha subunit, catalyzes synthesis of the second messenger cAMP from molecules of ATP. In humans, cAMP is involved in responses to sensory input, hormones, and nerve transmission, among others.
Phospholipase C is another common target of activated G proteins. Phospholipase C is a phosphodiesterase that hydrolyzes the membrane lipid phosphotidyl inositol to generate not one, but two second messengers — DAG and IP3. This particular pathway is critical to a wide variety of human bodily processes. For instance, thrombin receptors in platelets use this pathway to promote blood clotting. The seven-transmembrane (7TM) protein receptor in the plasma membrane activates a pathway that involves G proteins as well as cAMP-related pathways that modulate cellular signaling. Activated G alpha proteins inhibit (-) adenylyl cyclase, the enzyme that induces formation of cAMP, which in turn results in the activation of protein kinase A (PKA). This in turn activates a molecule called cAMP-responsive element-binding protein (CREB), which modulates gene transcription. GO proteins can also have a variety of other effects which include mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways. Activation of the enzyme phospholipase A2 (PLA2) may also occur, which induces the release of arachidonic acid (AA), as well as inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ levels. Subsequent activation of the MAPK and PI3K pathways results in the phosphorylation of extracellular signal-regulated kinases (ERKs) and protein kinase B (PKB), respectively. Activated PKB will subsequently phosphorylate and thereby inhibit the action of glycogen synthase kinase 3beta (GSK3beta), a major kinase in the brain.

References:
- Leurs, R. et al. (2005) The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nature Reviews Drug Discovery 4, 107-120.
- Bockaert J, Claeysen S, Be´camel Cet al. (2002) G protein-coupled receptors: dominant players in cell-cell communication. International Review of Cytology 212: 63–132.
- Li, J. et al. (2002) The Molecule Pages database. Nature 420, 716-717.
- Bockaert J and Pin JP (1999) Molecular tinkering of G protein coupled receptors: an evolutionary success. EMBO Journal18(7): 1723–1729.
- Bohm SK, Grady EF and Bunnett NW (1997) Regulatory mechanisms that modulate signalling by G- protein-coupled receptors. Biochemical Journal 322(part 1): 1–18
Published date : 14 Jun 2014 12:07PM










