Cell Cycle Regulation
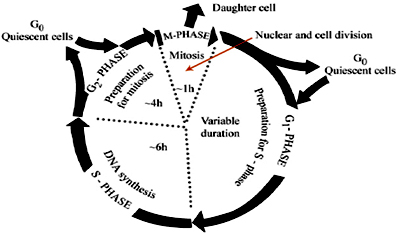
After cell division, each of the daughter cells begins the interphase of a new cycle. Although the various stages of interphase are not usually morphologically distinguishable, each phase of the cell cycle has a distinct set of specialized biochemical processes that prepare the cell for initiation of cell division. Neurons and skeletal muscle cells always remain in Go phase.
During G1, expression of genes associated with deoxynucleotidebiosynthesis is upregulated (e.g. thymidinekinase (TK), thymidylate synthase and dihydrofolatereductase (DFR)) in preparation for DNA synthesis. Ascells progress through G1, regulatory factors required forinitiation of DNA replication are sequentially expressedand/or activated. Following stimulation of quiescent cellsto proliferate, expression of the FOS/JUN-related earlyresponse genes is induced early in G1, playing a pivotal rolein activation of subsequent cell cycle regulatory events.
In S phase, DNA replication is paralleled by and functionallycoupled with histone gene expression, providing thenecessary basic chromosomal proteins (H1, H4, H3, H2A and H2B) for packaging newly replicated DNA into chromatin.
During G2, regulatory factors for mitosis are synthesised, andmodifications of chromatin structure to support mitoticchromosome condensation occur.
Mitosis involves asequential remodelling of genome architecture from looselypackaged chromatin to highly condensed chromosomesand back to chromatin; assembly and subsequent disassemblyof the mitotic apparatus; breakdown and reformationof the nuclear membrane; and modifications inactivities of factors required for reinitiation of cell cycleprogression, quiescence or differentiation. There is mitotic retention of transcription factors which supports the cellular capability to convey important information to progenycells to sustain physiological control of gene expression during proliferation. Mitotic association of transcription factors is facilitated by the focal organisationof a limited number of multiprotein complexes and nucleicacids that support physiological processes in subnuclear microenvironments (e.g. foci containing Runx proteins).
Regulation of eukaryotic cell cycle
Regulation of the cell cycle involves processes crucial to the survival of a cell, including the detection and repair of genetic damage as well as the prevention of uncontrolled cell division. The molecular events that control the cell cycle are ordered and directional; that is, each process occurs in a sequential fashion and it is impossible to "reverse" the cycle.
| State | Description | Abbreviation | |
| quiescent/ | G0 | A resting phase where the cell has left the cycle and has stopped dividing. | |
| G1 | Cells increase in size in Gap 1. | ||
| S | DNA replication occurs during this phase. | ||
| G2 | During the gap between DNA synthesis and mitosis, the cell will continue to grow. | ||
| M | Cell growth stops at this stage and cellular energy is focused on the orderly division into two daughter cells. A checkpoint in the middle of mitosis |
Role of Cyclins and CDKs
Two key classes of regulatory molecules, cyclins and cyclin-dependent kinases (CDKs), determine a cell's progress through the cell cycle. Leland H. Hartwell, R. Timothy Hunt, and Paul M. Nurse won the 2001 Nobel Prize in Physiology or Medicine for their discovery of these central molecule .Many of the genes encoding cyclins and CDKs are conserved among all eukaryotes.
Cyclins form the regulatory subunits and CDKs the catalytic subunits of an activated heterodimer; cyclins have no catalytic activity and CDKs are inactive in the absence of a partner cyclin. When activated by a bound cyclin, CDKs perform a common biochemical reaction called phosphorylation that activates or inactivates target proteins to orchestrate coordinated entry into the next phase of the cell cycle. Different cyclin-CDK combinations determine the downstream proteins targeted. CDKs are constitutively expressed in cells whereas cyclins are synthesised at specific stages of the cell cycle, in response to various molecular signals. In mammalian cells at least 16 cyclins and 9 CDKs have been identified.
There are two main groups of cyclins:
- G1/S cyclins – essential for the control of the cell cycle at the G1/S transition,
- Cyclin A / CDK2 – active in S phase.
- Cyclin D / CDK4, Cyclin D / CDK6, and Cyclin E / CDK2 – regulates transition from G1 to S phase.
- G2/M cyclins – essential for the control of the cell cycle at the G2/M transition (mitosis). G2/M cyclins accumulate steadily during G2 and are abruptly destroyed as cells exit from mitosis (at the end of the M-phase).
- Cyclin B / CDK1 – regulates progression from G2 to M phase.
The activities of the CDKs are down regulated by a series ofinhibitors (designated CKIs) and mediators of ubiquitination, which signal destabilisation and/or destruction ofthese regulatory complexes in a cell cycle-dependent manner.
Cell cycle regulatory factors mediating theG0/ G1, G1/S, S/G2 and G2/M transitions
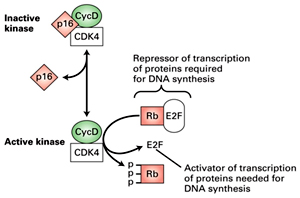 P16 is a cyclin kinase inhibitor-CKI.Mutation in p16 inactivates its ability to inhibit CDK activity
P16 is a cyclin kinase inhibitor-CKI.Mutation in p16 inactivates its ability to inhibit CDK activity- Cyclin D complexeswith CDK4 or CDK6 to phosphorylate pRB (retinoblastoma tumour suppressor protein). During early G1, the hypophosphorylated form of pRB binds and inactivates the E2F transcription factor. Following phosphorylation, E2F is released and transactivates a series of genes required for DNA replication.
- Progression through the late G1restriction point is controlled by a CDK2/cyclin E complex. Although activity of CDK2/cyclin E is required for the G1/S transition, cyclin E is not requisite for the progression of S phase.
- CDK2/cyclin A is required for the initiation of DNAreplication and to support DNA synthesis throughout Sphase. CDK2/cyclin A forms a quaternary complex with the p107 RB-related protein and E2F. In addition, cyclinA may play a role in cell adhesion and signal transductionfrom the plasma membrane.
- Recent findings suggest that the CDK4/cyclin A complex phosphorylates a single-stranded DNA-bindingprotein designated RPA34, which blocks rereplication of DNA.
- Histonegene transcription is activated at the G1/S phase transition and downregulated during quiescence or differentiation. One of the most critical factors that regulate histone H4 gene transcription is histone nuclear factor P (HiNF-P) and the concomitant recruitment of its coactivator p220NPAT, a cyclin E/CDK2 kinase substrate. This transcription factor binds specifically to the cell cycle regulatory element site II, a highly conserved region among multiple histone H4 genes. Depletion of HINFP protein impedes S phase progression and reduces histone H4 transcript.
- At the onset of mitosis, chromosome condensationis mediated by cyclin B/CDK1 complexes. Phosphorylationof histone H1 by CDK1/cyclin B modifies chromatinstructure.CDK1/cyclin B also contributes to chromosome condensation by phosphorylating and consequently activatingcaseine kinase which is the topoII activator. CDK1 hasbeen functionally linked to control of mitosis by phosphorylation-dependent abrogation of laminphosphorylation, which results in nuclear envelope breakdown. Additionally, both cyclin A/CDK1 and cyclin B/CDK1promote microtubule formation from centrosomes.
- During G2, CDK1 isinactivated by phosphorylation of Tyr15 by WEE1. Initiationof mitosis is functionally coupled to inactivation of WEE1 by phosphorylation, which is mediated by a series ofkinases that includes the NIM1 kinase.
Cyclins A and B are targeted for proteolysis is by ubiquitination in metaphase. Degradation of other cyclins is mediated by a PEST sequence (a protein degradation signal enriched in proline, glutamic acid, serine and threonine).
Cell cycle stage-specific and ubiquitindependentturnover of cell cycle regulatoryfactors
Apart from degradation of cyclins at specific stages during the cell cycle, ubiquitin-dependent proteolysis may also be importantfor regulating the activities of onco-proteins including c-Fos, c-Jun and IRF2.
The CDC34gene is essential for the G1/S phase transition. Ubiquitin dependent degradation is also involved in constitutive turnover of cyclins throughout G1phase.
Ubiquitin-dependent degradation also performs a keyregulatory function during the G2/M transition. The E2 enzymes encoded by UBC4 and UBC9 are involved indegradation of G1 and S cyclins before the onset of mitosis.
Similarly, completion of mitosis is regulated by the anaphase-promoting complex (APC). This high-molecular weight ubiquitination-complex is essential for sisterchromatid separation during chromosome segregation.
Role of p53 in cell cycle regulation
It is essential for G1 checkpoint control, arrests cells with damaged DNA in G1 hence called as the “guardian of the genome’ or gatekeeper.
Normally inhibits proliferation and stimulates apoptosis.It is expressed in low levels in normal cells.Stress leads to activation of certain kinases like ATM -Gene mutated in ataxia telangietasia and DNA dependent protein kinase. Phosphorylation of P53 by these kinases stabilises it leading to increase in its concentration –induces proteins that promotes apoptosis
P53 is Transcription factor-relevant to its tumor suppressing function. It induces the transcription of P21 (cyclin kinase inhibitor) which inhibits Cdk4-cyclinD ,cannot phosphorylate Rb-EF-2 bound to Rb- causes G1 arrest till the damage is repaired .This gives the cell time to either repair the DNA damage and then proceed into S phase or if the damage cannot be repaired the cell goes to apoptosis G2-M checkpoint is also governed by p53 but mechanism not yet known P53 is a tetrameric protein in mammalian cell’s stress response (DNA damage) ,a missense mutation in one of the alleles abrogates all activity.it is mutated in 50% of cancers.
Cell cycle regulation in plants
Plant genomes encode many different cyclin-dependent kinases (CDKs) and cyclin. The major drivers of the plant cell cycle are the A- and B-type CDKs.
A-type CDKs (CDKA) are most closely related to mammalian CDK1 and CDK2,they are constitutively present during the cell cycle, and they control the G1–S and G2–M transition points. A-type cyclins (CYCA) are mainly present from S phase to M phase,
B-type CDKs (CDKB) are specific to plants and accumulate in a manner that is dependent on the phase of the cell cycle, reaching a maximum level at the G2–M transition and during M phase.
The activity of the CDKA–CYCD complexes can be inhibited by their association with CDK inhibitory proteins (ICK/KRPand SIM) that respond to stress stimuli or developmental signals.
Selective destruction of CYCA and CYCB by the anaphase-promoting complex (APC) marks the exit from mitosis.
Transcriptional Control of Cell Cycle in Plants
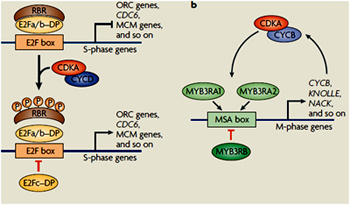 As in mammalian cells, plant E2Fs are activated following phosphorylation of the retinoblastoma-related (RBR) protein by the cyclin-dependent kinase (CDK)–D-type cyclin (CYCD) complexes. In Arabidopsis thaliana, E2Fa and E2Fb function as transcriptional activators and positive regulators of the cell cycle in a manner similar to that of the mammalian E2F1, E2F2 and E2F3. By contrast, E2Fc acts as a transcriptional repressor and suppressor of cell division. They havepivotal roles at the G1–S transition by modulating the expression of genes involved in DNA replication, cell-cycle progression and chromatin dynamics.
As in mammalian cells, plant E2Fs are activated following phosphorylation of the retinoblastoma-related (RBR) protein by the cyclin-dependent kinase (CDK)–D-type cyclin (CYCD) complexes. In Arabidopsis thaliana, E2Fa and E2Fb function as transcriptional activators and positive regulators of the cell cycle in a manner similar to that of the mammalian E2F1, E2F2 and E2F3. By contrast, E2Fc acts as a transcriptional repressor and suppressor of cell division. They havepivotal roles at the G1–S transition by modulating the expression of genes involved in DNA replication, cell-cycle progression and chromatin dynamics.MYB3R proteins are transcriptional activators that bind the M-specific activator (MSA) box in the promoter region of their target genes The MSA box is necessary and sufficient to drive G2–M-specific gene transcription. Three MYB3R proteins (MYB3RA1, MYB3RA2 and MYB3RB) that are structurally related to animal c-Myb proteins have been identified in tobaccoplants.
Inhibition of entry into mitosis by DNA damage in mammals versus plants-
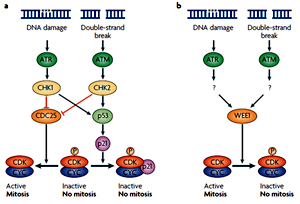 In mammals, the ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) kinases signal DNA stress during S phase to the checkpoint-1 (CHK1) and CHK2 protein kinases. CHK2 targets the CDC25 phosphatase for destruction or inhibition of its nuclear import. As a result, the cyclin-dependent kinase (CDK)–cyclin(CYC) complex is kept in a phosphorylated and inactive state, preventing mitotic entry.
In mammals, the ataxia-telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) kinases signal DNA stress during S phase to the checkpoint-1 (CHK1) and CHK2 protein kinases. CHK2 targets the CDC25 phosphatase for destruction or inhibition of its nuclear import. As a result, the cyclin-dependent kinase (CDK)–cyclin(CYC) complex is kept in a phosphorylated and inactive state, preventing mitotic entry.p21, which is transcriptionally induced by the tumour suppressor protein p53, can also inhibit CDK activity.
Plants share the ATR and ATM kinases with mammals, but lack clear homologues of CHK1 and CHK2. Moreover, because of the absence of a functional CDC25 in plants, the transcriptional induction of the antagonistic WEE1 kinase gene represents the prevailing pathway for arrest of the cell cycle in response to DNA stress. The molecular pathway that connects WEE1 induction with ATR and ATM is still unknown.
References:
- Malumbres M, Harloe E, Hunt F et al. (2009) Cyclin Dependent Kinases: A Family Portrait. Nature Cell Biology 11(11); 1275-1276.
- Zaidi SK, Young DW, Javed .A et al. (2007) Nuclear Microenvironments in Biological Control and Cancer. Nature reviews Cancer 7; 454-463.
- Zaidi SK, Young DW, Montecino M et al. (2010) Mitotic Book Marking of Genes; a Novel Dimension to Epigenetic Control. Nature reviews Genetics 11(8); 583-589.
- Jakoby M and Schnittger A (2004) Cell Cycle and Differentiation. Current Opinion in Plant Biology 7; 661-669.
- Dirk Inze et al. (2007) The Ins and Outs of the Plant Cell Cycle. Nature Reviews: Molecular Cell Biology 8; 655-665.
- Clive Lloyd and Jordi Chan (2006) Not so Divided: the Common Basis of Plant and Animal Cell Division. Nature reviews Molecular Cell Biology 7; 147-152.










