Amino Acids
Sakshi Education
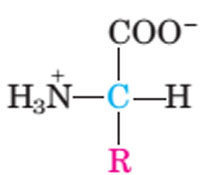
In general any compound, natural or synthetic, with an amino group and carboxylic acid group can be termed as an Amino acid. In this manner there would be innumerable number of amino acids. Naturally, there are about more than 300 amino acids present in different living systems (animal, plant and microbial systems). What we refer here are amino acids that are frequently observed in proteins and contain a codon in the DNA sequences. Such amino acids are termed as “Standard Amino acids”. There are 22 amino acids that appear in mammalian proteins. 20 amino acids are familiar, 21st amino acid is Selenocysteine and 22 is polylysine. All proteins are composed of 20 “standard” amino acids. Not every protein contains all 20 types of amino acids, but most proteins contain most if not all of the 20 types. The first amino acid to be discovered was asparagines found in asparagus, in 1806 and Threonine being the last of the twenty.
The common amino acids are known as a-amino acids because they have a primary amino group (-NH2) on the a-carbon as a substituent, Along with other functional group are…
Twenty amino acids are encoded by the standard genetic code and are called proteinogenic or standard amino acids. The mean mass of the standard amino acid residues, weighed by abundance in proteins, is roughly 110 Daltons. Combinations of these amino acids produce every single essential protein for the homeostasis of the human body.
Classification:
The properties of each amino acid are dependent on its side chain (—R); the side chains are the functional groups that are the major determinants of the structure and function of proteins, as well as the electrical charge of the molecule. Knowledge of the properties of these side chains is important for understanding methods of analysis, purification, and identification of proteins. The amino acid of R groups, vary in structure, size, and electric charge influencing the physico-chemical properties of amino acids like solubility of the amino acids in water. The nature of the side chains that ultimately dictates the role of an amino acid in proteins. According to the most common classification scheme, there are three major types of amino acids:
(1) those with nonpolar R group,
(2) those with uncharged polar R groups, and
(3) those with charged polar R groups.
Amino acids with Non-Polar R group- (Glycine, Alanine, Valine, Leucine and Isoleucine, phynylalanine, tryptophane, methionine, proline).
Each of these amino acids has a nonpolar side chain that does not bind or release protons, or participate in hydrogen or ionic bonds. The side chains of these amino acids can be thought of as "oily" or lipid-like, a property that promotes hydrophobic interactions.
Examples of nonstandard amino acids that are not found in proteins (Non protein amino acids) include lanthionine, 2-aminoisobutyric acid, the sulfur-containing taurine, dehydroalanine, dopamine (melanin precursor) and the neurotransmitter gamma-aminobutyric acid. Nonstandard amino acids often occur as intermediates in the metabolic pathways for standard amino acids - for example, ornithine and citrulline occur in the urea cycle, part of amino acid catabolism.
Some of the amino acids act as:
The common amino acids are known as a-amino acids because they have a primary amino group (-NH2) on the a-carbon as a substituent, Along with other functional group are…
- an acidic carboxyl group ( —COOH)
- a hydrogen atom ( —H)
- a distinctive side chain ( —R).
General structure of amino acid is:
Above structure is common for all amino acids but the sole exception is proline, which has a secondary amino group (-NH), although for uniformity we refer to proline as an a-aminoacid but it is a a -imino acid.
Optical Properties
With the exception of glycine, all the amino acids recovered from polypeptides are optically active; that is, they rotate the plane of polarized light. The direction and angle of rotation can be measured using an instrument known as a polarimeter. Except for glycine, all amino acids contain at least one asymmetric carbon atom (the a -carbon atom), giving two stereoisomer’s that are optically active.
Structurally, stereoisomers are defined as non-superimposable chemical isomers that have identical covalent structures. There are two classes of stereoisomerisam, namely -
i). Enantiomers, and
ii). Diastereoisomers
Enantiomers are mirror images chemical isomers.
Diastereoisomers are non-mirror images chemical isomers.
Every amino acid (except glycine) can occur in two isomeric forms, because of the possibility of forming two different enantiomers (stereoisomers) around the central carbon atom. By convention, these are called L- and D- forms, analogous to left-handed and right-handed configurations respectively, but Only L-amino acids are manufactured in cells and incorporated into proteins because proteins are biosynthesized by enzymes that insert only L- amino acids into the peptide chains. Some D-amino acids are found in antibiotics and the cell walls of bacteria, but not in bacterial proteins (The presence of these D isomers protects the bacteria from enzymes, the host organism uses to protect itself from bacterial infection by hydrolyzing the proteins in the bacterial cell wall.). These D- amino acids are directly joined together by the action of specific bacterial enzyme but not translation machinery. Some of the D- amino acid found in bacteria mentioned in the table.
Above structure is common for all amino acids but the sole exception is proline, which has a secondary amino group (-NH), although for uniformity we refer to proline as an a-aminoacid but it is a a -imino acid.
Optical Properties
With the exception of glycine, all the amino acids recovered from polypeptides are optically active; that is, they rotate the plane of polarized light. The direction and angle of rotation can be measured using an instrument known as a polarimeter. Except for glycine, all amino acids contain at least one asymmetric carbon atom (the a -carbon atom), giving two stereoisomer’s that are optically active.
Structurally, stereoisomers are defined as non-superimposable chemical isomers that have identical covalent structures. There are two classes of stereoisomerisam, namely -
i). Enantiomers, and
ii). Diastereoisomers
Enantiomers are mirror images chemical isomers.
Diastereoisomers are non-mirror images chemical isomers.
Every amino acid (except glycine) can occur in two isomeric forms, because of the possibility of forming two different enantiomers (stereoisomers) around the central carbon atom. By convention, these are called L- and D- forms, analogous to left-handed and right-handed configurations respectively, but Only L-amino acids are manufactured in cells and incorporated into proteins because proteins are biosynthesized by enzymes that insert only L- amino acids into the peptide chains. Some D-amino acids are found in antibiotics and the cell walls of bacteria, but not in bacterial proteins (The presence of these D isomers protects the bacteria from enzymes, the host organism uses to protect itself from bacterial infection by hydrolyzing the proteins in the bacterial cell wall.). These D- amino acids are directly joined together by the action of specific bacterial enzyme but not translation machinery. Some of the D- amino acid found in bacteria mentioned in the table.
| Amino acid | Occurance |
| D-Phenyl alanine | Gramsidin |
| D-Valine | Actinomycin- D |
| D-Alanine & D-Glutamic acid | Peptidoglycan of Gram +ve |
Twenty amino acids are encoded by the standard genetic code and are called proteinogenic or standard amino acids. The mean mass of the standard amino acid residues, weighed by abundance in proteins, is roughly 110 Daltons. Combinations of these amino acids produce every single essential protein for the homeostasis of the human body.
Classification:
The properties of each amino acid are dependent on its side chain (—R); the side chains are the functional groups that are the major determinants of the structure and function of proteins, as well as the electrical charge of the molecule. Knowledge of the properties of these side chains is important for understanding methods of analysis, purification, and identification of proteins. The amino acid of R groups, vary in structure, size, and electric charge influencing the physico-chemical properties of amino acids like solubility of the amino acids in water. The nature of the side chains that ultimately dictates the role of an amino acid in proteins. According to the most common classification scheme, there are three major types of amino acids:
(1) those with nonpolar R group,
(2) those with uncharged polar R groups, and
(3) those with charged polar R groups.
Amino acids with Non-Polar R group- (Glycine, Alanine, Valine, Leucine and Isoleucine, phynylalanine, tryptophane, methionine, proline).
Each of these amino acids has a nonpolar side chain that does not bind or release protons, or participate in hydrogen or ionic bonds. The side chains of these amino acids can be thought of as "oily" or lipid-like, a property that promotes hydrophobic interactions.
Location of nonpolar amino acids in proteins
In proteins found in aqueous solutions, the side chains of the nonpolar amino acids tend to cluster together in the interior of the protein. This phenomenon is due to the hydrophobicity of the nonpolar R-groups, which act much like droplets of oil that coalesce in an aqueous environment. The nonpolar R-groups thus fill up the interior of the folded protein, and help give it its three-dimensional shape. (In proteins that are located in a hydrophobic environment such as a membrane, the nonpolar R-groups are found on the surface of the protein, interacting with the lipid environment These hydrophobic interactions are the key forces that stabilize protein structure.
Proline; The side chain of proline and its a-amino group form a distinctive cyclic structure. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline (causing abrupt changes in the direction of the polypeptide chain.)
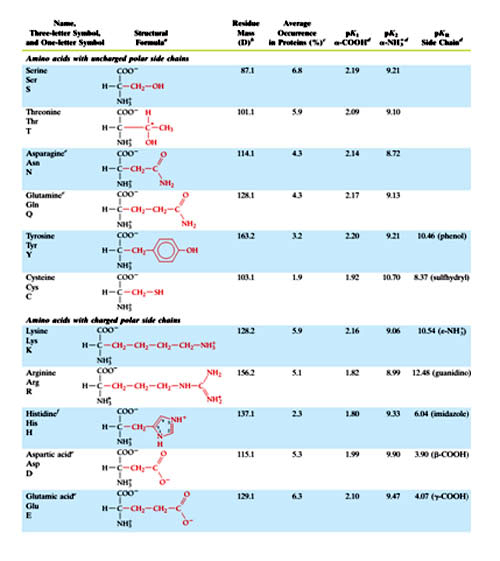
Amino acids with uncharged polar side chains
These amino acids have zero net charge at neutral pH, although the side chains of cysteine and tyrosine can lose a proton at an alkaline pH. Serine, threonine, and tyrosine each contain a polar hydroxyl group that can participate in hydrogen bond formation. The side chains of asparagine and glutamine each contain a carbonyl group and an amide group, both of which can also participate in hydrogen bonds.
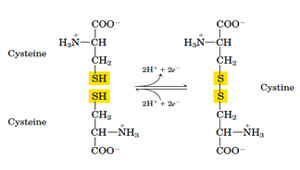
Disulfide bond: Cysteine and its oxidized form, cystine, are sulfur-containing amino acids characterized by low polarity. Cysteine plays an important role in stabilization of protein structure and cross-linking protein chain, since it can participate in formation of a disulfide bond with other cysteine residues to form cystine residues. Two regions of a single polypeptide chain, remote from each other in the sequence, may be covalently linked through a disulfide bond (intrachain disulfide bond). Disulfide bonds are also formed between two polypeptide chains (interchain disulfide bond), forming covalent protein dimers. These bonds can be reduced by enzymes or by reducing agents such as 2-mercaptoethanol or dithiothreitol, to form cysteine residues.
Side chains as sites of attachment for other compounds:
In proteins found in aqueous solutions, the side chains of the nonpolar amino acids tend to cluster together in the interior of the protein. This phenomenon is due to the hydrophobicity of the nonpolar R-groups, which act much like droplets of oil that coalesce in an aqueous environment. The nonpolar R-groups thus fill up the interior of the folded protein, and help give it its three-dimensional shape. (In proteins that are located in a hydrophobic environment such as a membrane, the nonpolar R-groups are found on the surface of the protein, interacting with the lipid environment These hydrophobic interactions are the key forces that stabilize protein structure.
Proline; The side chain of proline and its a-amino group form a distinctive cyclic structure. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline (causing abrupt changes in the direction of the polypeptide chain.)
Amino acids with uncharged polar side chains

These amino acids have zero net charge at neutral pH, although the side chains of cysteine and tyrosine can lose a proton at an alkaline pH. Serine, threonine, and tyrosine each contain a polar hydroxyl group that can participate in hydrogen bond formation. The side chains of asparagine and glutamine each contain a carbonyl group and an amide group, both of which can also participate in hydrogen bonds.
Disulfide bond: Cysteine and its oxidized form, cystine, are sulfur-containing amino acids characterized by low polarity. Cysteine plays an important role in stabilization of protein structure and cross-linking protein chain, since it can participate in formation of a disulfide bond with other cysteine residues to form cystine residues. Two regions of a single polypeptide chain, remote from each other in the sequence, may be covalently linked through a disulfide bond (intrachain disulfide bond). Disulfide bonds are also formed between two polypeptide chains (interchain disulfide bond), forming covalent protein dimers. These bonds can be reduced by enzymes or by reducing agents such as 2-mercaptoethanol or dithiothreitol, to form cysteine residues.
Side chains as sites of attachment for other compounds:

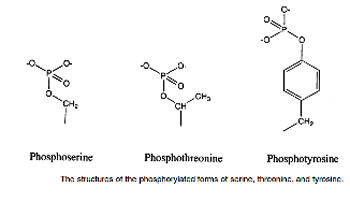
Serine, threonine, and, rarely, tyrosine contain a polar hydroxyl group that can serve as a site of attachment, for structures such as a phosphate group. (The side chain of serine is an important component of the active site of many enzymes.) In addition, the amide group of asparagine, as well as the hydroxyl group of serine or threonine, can serve as a site of attachment of oligosaccharide chains in glycoproteins .
Amino acids with Positively Charged (Basic) R Groups
The side chains of the basic amino acids accept protons. At physiological pH the side chains of lysine and arginine are fully ionized and positively charged. The epsilon- amino group of lysine has a p Ka ~ 11. Arginine is the most basic amino acid (p Ka ~ 13) In contrast, histidine (p K a ~ 6) is weakly basic and has an imidazole group, and the free amino acid is largely unchanged at physiologic pH(7.4). However, when histidine is incorporated into a protein, its side chain can be either positively charged or neutral, depending on the ionic environment provided by the polypeptide chains of the protein. This residue facilitates the reaction by serving as a proton donor/acceptor and hence is found in the active site of the enzymes mostly frequently.
Amino acids with Negatively Charged (Acidic) R Groups
Aspartic and glutamic acids contain carboxylic acids on their side chains and are ionized at pH 7.0 and, as a result, carry negative charges on their ß - and ? -carboxyl groups, respectively. In the ionized state, these amino acids are referred to as aspartate and glutamate, respectively.
For tryptophan, absorption is maximum at 280 nm whereas, for tyrosine, absorption is maximum at 276 nm and phenylalanine absorbs light less strongly and at shorter wavelengths. The absorption of light at 280 nm can be used to estimate the concentration of a protein in solution if the number of tryptophan and tyrosine residues in the protein is known.
Abbreviations and symbols for the commonly occurring amino acids
Each amino acid name has an associated three-letter abbreviation and a one-letter symbol the one-letter codes are determined by the following rules:
Amino acids with Positively Charged (Basic) R Groups
The side chains of the basic amino acids accept protons. At physiological pH the side chains of lysine and arginine are fully ionized and positively charged. The epsilon- amino group of lysine has a p Ka ~ 11. Arginine is the most basic amino acid (p Ka ~ 13) In contrast, histidine (p K a ~ 6) is weakly basic and has an imidazole group, and the free amino acid is largely unchanged at physiologic pH(7.4). However, when histidine is incorporated into a protein, its side chain can be either positively charged or neutral, depending on the ionic environment provided by the polypeptide chains of the protein. This residue facilitates the reaction by serving as a proton donor/acceptor and hence is found in the active site of the enzymes mostly frequently.
Amino acids with Negatively Charged (Acidic) R Groups
Aspartic and glutamic acids contain carboxylic acids on their side chains and are ionized at pH 7.0 and, as a result, carry negative charges on their ß - and ? -carboxyl groups, respectively. In the ionized state, these amino acids are referred to as aspartate and glutamate, respectively.
For tryptophan, absorption is maximum at 280 nm whereas, for tyrosine, absorption is maximum at 276 nm and phenylalanine absorbs light less strongly and at shorter wavelengths. The absorption of light at 280 nm can be used to estimate the concentration of a protein in solution if the number of tryptophan and tyrosine residues in the protein is known.
Abbreviations and symbols for the commonly occurring amino acids
Each amino acid name has an associated three-letter abbreviation and a one-letter symbol the one-letter codes are determined by the following rules:
- Unique first letter: If only one amino acid begins with a particular letter, then that letter is used as its symbol. For example, I = isoleucine.
- Most commonly occurring amino acids have priority: If more than one amino acid begins with a particular letter, the most common of these amino acids receives this letter as its symbol. For example, glycine is more common than glutamate, so G = glycine.
- Similar sounding names: Some one-letter symbols sound like the amino acid they represent. For example, F = phenylalanine, or W = tryptophan (“twyptophan” as Elmer Fudd would say).
- Letter close to initial letter: For the remaining amino acids, a one letter symbol is assigned that is as close in the alphabet as possible to the initial of the amino acid. Further, B is assigned to Asx, signifying either aspartic acid or asparagine; Z is assigned to Glx, signifying either glutamic acid or glutamine; and X is assigned to an unidentified amino acid.
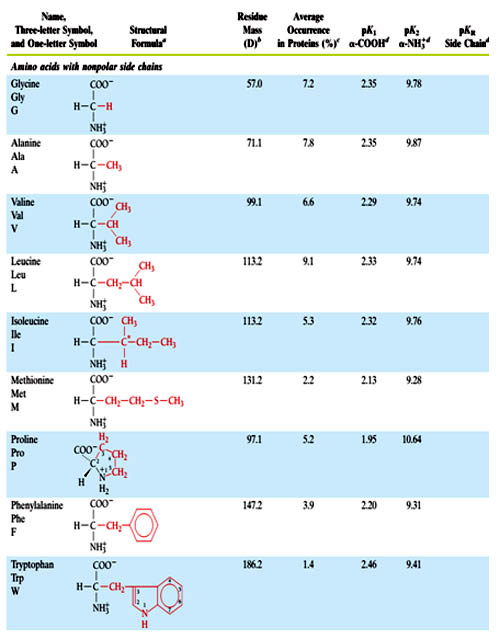
Covalent Structures and Abbreviations of the “Standard” Amino Acids of Proteins, their Occurrence, and the pK Values of Their Ionizable Groups in table 1
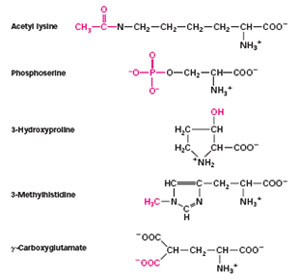
At least two others are also coded by DNA in a non-standard manner as follows:
Aside from the twenty standard amino acids, there are a vast number of "Nonstandard amino acids". These are inserted in to the protein by the translation machinery. Two of these as mentioned earlier can be encoded in the genetic code, but are rather rare in proteins.


At least two others are also coded by DNA in a non-standard manner as follows:
Aside from the twenty standard amino acids, there are a vast number of "Nonstandard amino acids". These are inserted in to the protein by the translation machinery. Two of these as mentioned earlier can be encoded in the genetic code, but are rather rare in proteins.
- Selenocysteine is incorporated into some proteins at a UGA codon, which is normally a stop codon. It is a structural analog to cysteine but contains selenium rather than sulphur. It has a cognate t-RNA during protein translation containing the anti-codon UCA. Enzymes like glutathione peroxidase and formate dehydrogenase contain selenocysteine in their catalytic site.
- Pyrrolysine is used by some methanogenic bacteria in enzymes that they use to produce methane. It is coded for with the codon UAG. It is similar to lysine.
- Hydroxylysine and hydroxyproline (made by a posttranslational modification). These are found in the protein collagen. Collagen is a fibrous protein made up of three polypeptides that form a stable assembly, but only if the proline and lysine residues are hydroxylated. (requires vitamin C for reduction of these amino acids to hydroxy form)
- Thyroxine, an iodinated derivative of tyrosine, found in thyroglobulin (produced by thyroid gland; requires iodine in diet)
- ?-carboxyglutamic acid (i.e. glutamic acid with two carboxyl groups) found in certain blood clotting enzymes (requires vitamin K for production)
- N-methyl arginine and n-acetyl lysine. Found in some DNA binding proteins known as histones.
| Non- standard aminoacid | Occurrence | |
| 4-Hydroxy proline | Plant cell wall proteins & collagen | |
| 5-Hydroxy proline | Plant cell wall, fibrous protein, and collagen of connective tissue. | |
| N-Methyllysine | Myosin | |
| ?-Carboxy glutamate | Prothrombin | |
| Desmosin | Elastin | |
Examples of nonstandard amino acids that are not found in proteins (Non protein amino acids) include lanthionine, 2-aminoisobutyric acid, the sulfur-containing taurine, dehydroalanine, dopamine (melanin precursor) and the neurotransmitter gamma-aminobutyric acid. Nonstandard amino acids often occur as intermediates in the metabolic pathways for standard amino acids - for example, ornithine and citrulline occur in the urea cycle, part of amino acid catabolism.
Some of the amino acids act as:
- Tryptophan is a precursor of the neurotransmitter serotonin
- Glycine is a precursor of porphyrins such as heme
- Arginine is a precursor of the hormone nitric oxide
- Carnitine is used in lipid transport within a cell,
- Ornithine and S-adenosylmethionine are precursors of polyamines, (The polyamines are organic compounds having two or more primary amino groups - such as putrescine, cadaverine, spermidine, and spermine - that are growth factors in both eucaryotic and procaryotic cells)
- Homocysteine is an intermediate in S-adenosylmethionine recycling
- Serotonin (derivative of tryptophan) and g-amino butyric acid (a derivative of glutamic acid) are both neurotransmitters
- Histamine (derivative of histidine) involved in allergic response
- Adrenaline (derivative of tyrosine) a hormone
- Various antibiotics are amino acid derivatives (penicillin).
- Aspartame (aspartyl-phenylalanine-1-methyl ester) is an artificial sweetener.
- 5-HTP (5-hydroxytryptophan) has been used to treat neurological problems associated with PKU (phenylketonuria), as well as depression (as an alternative to L-Tryptophan).
- L-DOPA (L-dihydroxyphenylalanine) is a drug used to treat Parkinsonism.
- Monosodium glutamate is a food additive to enhance flavor.
Covalent Modifications of Amino acids:
The covalent attachment of another molecule can modify the activity of enzymes and many other proteins. Most modifications are reversible.
Acetylation: The addition of an acetyl group to the amino group of the N-terminal residue is the most common form of chemical modification, affecting an estimated 80 percent of all proteins. This modification may play an important role in controlling the life span of proteins within cells because nonacetylated proteins are rapidly degraded by intracellular proteases.
Phosphorylation: An important modification is the phosphorylation of serine, threonine, tyrosine, and histidine residues. We will come across numerous examples of proteins whose activity is regulated by reversible phosphorylation and dephosphorylation.
Glycosylation: The side chains of asparagines, serine, and threonine are sites for glycosylation, the attachement of linear and branched carbohydrate chains. Many secreted proteins and membrane proteins contain glycosylated residues.
Modification is not readily reversible in some cases. Some proteins in signal-transduction pathways, such as Ras and Src (a protein tyrosine kinase), are localized to the cytoplasmic face of the plasma membrane by the irreversible attachment of a lipid group. Having fixed in such cellular location, the proteins can receive and transmit information that is being passed along their signaling pathwaysin a better way.
The attachment of ubiquitin, a protein comprising 72 amino acids, acts as a signal that marks a protein for degradation, the ultimate means of regulation. Cyclin, an important protein in cell-cycle regulation must be ubiquitinated and destroyed before a cell can enter anaphase and proceed through the cell cycle.
Essential and non essential amino acids:
Some of the 20 standard proteinogenic amino acids are called essential amino acids as the human body cannot synthesize them from other compounds through chemical reactions, and are therefore required to be obtained from food. Histidine and arginine are generally only considered essential in children, because the metabolic pathways that synthesize these amino acids are not fully developed in children.
Essential: Histidine*, Arginine*, Leucine, Lysine, Valine, Isoleucin, Methionine, Phenylalanine, Tryptophan, Threonine (code: MILK F TV W)
Nonessential: Tyrosine, Glycine, Alanine, Glutamine, Asparagine, Glutamic acid, Aspartate, Proline, Cysteine, Serine.
Properties of amino acids:
Acid-Base Properties of the Amino Acids
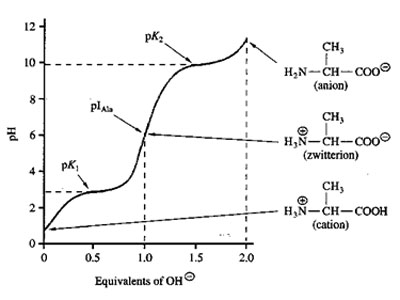
Amino acids are amphoteric molecules – they have both basic and acidic groups. Monoamino and monocarboxylic acids are ionized in different ways in solution, depending on the solution’s pH. Molecules such as amino acids, which bear charged groups of opposite polarity, are known as dipolar ions or zwitterions. As a consequence of their internal charge separations, zwitterions possess large dipole moments. At pH 7, the a-COOH and a -NH2 group in amino acids are capable of ionizing. As a result of their ionizability the following ionic equilibrium reactions may be written:
R-COOH <--------> R-COO- + H+
R-NH3+ <---------> R-NH2 + H+
The equilibrium reactions, as written, demonstrate that amino acids contain at least two weakly acidic groups. However, the carboxyl group is a far stronger acid than the amino group. At physiological pH (around 7.4) the carboxyl group will be unprotonated and the amino group will be protonated. An amino acid with no ionizable R-group would be electrically neutral at this pH. This species is termed a zwitterion.
The net charge (the algebraic sum of all the charged groups present) of any amino acid, peptide or protein, will depend upon the pH of the surrounding aqueous environment. As the pH of a solution of an amino acid or protein changes so too does the net charge. This phenomenon can be observed during the titration of any amino acid or protein. When the net charge of an amino acid or protein is zero the pH will be equivalent to the isoelectric point: pI. Above the pI valve the charge of the amino acid is negative and below the pI value the charge of the amino acid is positive.
pI is the arithmetic means of the two pka values - pI = ½ (pk1+pk2)
For acidic amino acids - pI = ½(pka1+pka2)
For basic aminoacids – pI = ½(pkb1+pkb2)
References:

The covalent attachment of another molecule can modify the activity of enzymes and many other proteins. Most modifications are reversible.
Acetylation: The addition of an acetyl group to the amino group of the N-terminal residue is the most common form of chemical modification, affecting an estimated 80 percent of all proteins. This modification may play an important role in controlling the life span of proteins within cells because nonacetylated proteins are rapidly degraded by intracellular proteases.
Phosphorylation: An important modification is the phosphorylation of serine, threonine, tyrosine, and histidine residues. We will come across numerous examples of proteins whose activity is regulated by reversible phosphorylation and dephosphorylation.
Glycosylation: The side chains of asparagines, serine, and threonine are sites for glycosylation, the attachement of linear and branched carbohydrate chains. Many secreted proteins and membrane proteins contain glycosylated residues.
Modification is not readily reversible in some cases. Some proteins in signal-transduction pathways, such as Ras and Src (a protein tyrosine kinase), are localized to the cytoplasmic face of the plasma membrane by the irreversible attachment of a lipid group. Having fixed in such cellular location, the proteins can receive and transmit information that is being passed along their signaling pathwaysin a better way.
The attachment of ubiquitin, a protein comprising 72 amino acids, acts as a signal that marks a protein for degradation, the ultimate means of regulation. Cyclin, an important protein in cell-cycle regulation must be ubiquitinated and destroyed before a cell can enter anaphase and proceed through the cell cycle.
Essential and non essential amino acids:
Some of the 20 standard proteinogenic amino acids are called essential amino acids as the human body cannot synthesize them from other compounds through chemical reactions, and are therefore required to be obtained from food. Histidine and arginine are generally only considered essential in children, because the metabolic pathways that synthesize these amino acids are not fully developed in children.
Essential: Histidine*, Arginine*, Leucine, Lysine, Valine, Isoleucin, Methionine, Phenylalanine, Tryptophan, Threonine (code: MILK F TV W)
Nonessential: Tyrosine, Glycine, Alanine, Glutamine, Asparagine, Glutamic acid, Aspartate, Proline, Cysteine, Serine.
Properties of amino acids:
Acid-Base Properties of the Amino Acids

Amino acids are amphoteric molecules – they have both basic and acidic groups. Monoamino and monocarboxylic acids are ionized in different ways in solution, depending on the solution’s pH. Molecules such as amino acids, which bear charged groups of opposite polarity, are known as dipolar ions or zwitterions. As a consequence of their internal charge separations, zwitterions possess large dipole moments. At pH 7, the a-COOH and a -NH2 group in amino acids are capable of ionizing. As a result of their ionizability the following ionic equilibrium reactions may be written:
R-COOH <--------> R-COO- + H+
R-NH3+ <---------> R-NH2 + H+
The equilibrium reactions, as written, demonstrate that amino acids contain at least two weakly acidic groups. However, the carboxyl group is a far stronger acid than the amino group. At physiological pH (around 7.4) the carboxyl group will be unprotonated and the amino group will be protonated. An amino acid with no ionizable R-group would be electrically neutral at this pH. This species is termed a zwitterion.
The net charge (the algebraic sum of all the charged groups present) of any amino acid, peptide or protein, will depend upon the pH of the surrounding aqueous environment. As the pH of a solution of an amino acid or protein changes so too does the net charge. This phenomenon can be observed during the titration of any amino acid or protein. When the net charge of an amino acid or protein is zero the pH will be equivalent to the isoelectric point: pI. Above the pI valve the charge of the amino acid is negative and below the pI value the charge of the amino acid is positive.
pI is the arithmetic means of the two pka values - pI = ½ (pk1+pk2)
For acidic amino acids - pI = ½(pka1+pka2)
For basic aminoacids – pI = ½(pkb1+pkb2)
References:
- Lehninger Principles of Biochemistry by Albert L. Lehninger.
- Fundamentals of Biochemistry by Donald Voet, Judith G. Voet.
- Biochemistry by Lubert Stryer
Published date : 15 May 2014 04:14PM










