Plant Tissue Culture
Sakshi Education

Introduction
The history of plant tissue culture dates back to1904, where Hannig isolated immature embryos in vitro from several brassicacae members and recovered fertile plants. Discovery of plant tissue culture medium, MS medium by US plant physiologists Murashige and Skoog in 1962 sped up the developments in the tissue culture techniques. Since then the science of plant tissue culture developed to its present state. The plant tissue culture techniques are based in part upon the ability of plants to reproduce asexually (vegetative reproduction) and completely on cell competence and totipotency, i.e. the ability of plant cells to express their genetic potential, to undergo dedifferentiation and redifferentiation, and to regenerate whole plant.
However all the plant cells do not have the ability to develop complete plant. Plant cell and tissue culture includes the techniques involved in the culture of aseptically isolated cells, protoplasts, tissues, and organs on a defined sterile medium under controlled environmental conditions. The regeneration of new plant may be either by organogenesis – where unipolar organ formation takes place or by somatic embryogenesis – where bipolar embryonic axis forms.
Plant tissue culture techniques serves as a valuable tool for studying the cell physiology and metabolism, this forms the basis for a major practical application, i.e., the production of secondary metabolites – phytochemicals for the food and pharmaceutical industries.
Propagation in plant tissue culture is used to develop high quality pathogen free plants. Micropropagation has an advantage of rapid, large scale multiplication of new genotypes and generation of pathogen free plants over conventional propagation.
The outstanding progress and remarkable achievements in plant tissue culture techniques complemented with developments in molecular biology, including embryo rescue, haploid production, somatic cells hybridization and genetic transformation facilitated the production of novel plant crops. Plant transformation relies on tissue culture methods to generate new crop plants for crop improvement. Transformation, introduction of DNA into plant cells was achieved either by biological vector like Agrobacterium or virus or by physical means like particle bombardment or chemical techniques like PEG mediated protoplast transformation. Each method has its own merits as well as demerits which should be considered along with the crop and explants to be transformed.
Regeneration of haploid cells and plants from isolated microspores enabled the production of homozygous plants for nonconventional breeding. These homozygous plants could be used to generate double haploids by doubling the chromosome number with in short time which otherwise takes a long time through conventional breeding program. Isolation and culture of protoplast has enabled us to study the plant cell wall apart from providing a system for somatic hybridization through protoplast fusion to overcome interspecific crossings barriers. Culture of young embryos allowed embryo rescue techniques used to overcome embryo abortion resulting from incompatible crossings of several species.
Isolated plant cell or organ, or tissue, used to initiate an in vitro culture is called as explant. Plant tissue culture techniques can be broadly divided into organized and unorganized cultures, based on the morphology of the established cultures. The term unorganized cultures is more general and includes all cell cultures where as organized cultures includes organ cultures in which some form of organized growth is maintained, as in the continued growth of shoot or root apices.
Organized cultures may contain mixtures of single cells and unorganized cell clumps, as well as organized structures. Similarly, unorganized cells can also be induced to organize and regenerate plant organs through organogenesis or embryogenesis.
Regeneration by organogenesis or embryogenesis can take place either directly from isolated cells, tissues, or organs or from an intermediate unorganized mass of cells called the callus/tissue phase. Direct regeneration may occur from isolated tissues or organs that regenerate shoots or roots, or from cells induced to form embryos. Indirect regeneration occurs following the proliferation of unorganized cells established in tissue (callus) or cell suspension culture, and may also occur from semiorganized callus or nodules produced on pieces of tissue or organ isolated in culture.
Factors which could have a play in plant tissue culture
Plant tissue culture techniques are very sensitive and greatly affected by internal environment of the explants as well as external environment. Success in tissue culture is greatly determined by nutrient media used for in vitro culture, conditions of in vitro culture and the plant material used as a source of explants.
Nutrient medium consists of minerals, vitamins, carbohydrates, plant growth regulators and a solidifying gelling agent in case of semisolid cultures. Each component of nutrient medium has an immense effect on the culture and proper care should be taken for selecting specific medium for specific explants and crop species.
Temperature and the amount of light and duration have either inhibiting or enhancing effect on the response of plant tissue in vitro and needed to be adapted for each plant or tissue requirements. Usually cultures are kept under low light intensities. The physiological status of the tissue has a paramount role in response of isolated cultured explants. Usually juvenile and young actively growing tissues respond better to hormonal signals than older tissues in culture medium.
Laboratory Facilities
The facilities of tissue culture laboratories are organized into three major areas, one is used for all nonaseptical chores like media preparation and autoclaving and for initial plant preparation. The second area is used for performing all the aseptical procedures of explant isolation, sterilizatrion, planting and for subculturing the cultures in a laminar air hood. The third area is used for maintenance of the cultures under controlled temperature and light quality and duration as well as humidity as specified for each plant culture.
Nutrient Medium
Success of plant tissue culture is greatly dependent on selection of the culture medium. Typical, culture media includes minerals supplied in the form of their salts for providing macro and micro nutrients, classified based on their requirement. It also includes a carbon source, usually sucrose. Apart from the essential ingredients culture media also includes vitamins and amino acids. Culture media also includes the most vital compounds called as plant growth hormones, which in very low concentrations can alter the plant growth and development (morphology).
Many specific culture media formulations are developed for different stages of culture progression or for individual species, of all the media formulations available till date one developed by Murashige and Skoog is widely used. Plant material can be cultured in a liquid medium or in a semisolid medium partially solidified with a gelling agent such as agar or gellan gum. Cultures grown in a semisolid medium are kept static, whereas liquid cultures are usually agitated to ensure adequate gaseous exchange.
Components of Nutrient medium and their role
Media component Role/Significance
Macroelements
Calcium (Ca) Cofactor for many enzymes and cell wall synthesis
Magnesium (Mg) Cofactor for enzymes, integral component of chlorophyll molecule and cation in plants to balance negative ions
Nitrogen (N) Constituent of amino acids, proteins, nucleic acid, certain hormones and chlorophyll
Phosphorus (K) Integral part of nucleic acids and other structural compounds in plants
Potassium (K) Major positive ion to balance negative ion in plants
Sulphur (S) Important in disulphide bond formation in tertiary protein structure. Constituent of the vitamins thiamine and biotin and of coenzyme A, which has a key role in fatty acid metabolism and electron transfer reactions of photosynthesis
Iron (Fe) Required for chlorophyll synthesis and in many oxidation and reduction reactions. Iron is added in chelated form as ferrous-EDTA to prevent precipitation
The Microelements
Boron (B) Essential in enzyme activity in lignin biosynthesis and metabolism of phenolic acids. Deficiency inhibits cell division and elongation in root tip and shoot tip meristems
Cobalt (Co) Not considered as essential element, may be toxic at higher concentrations
Copper (Cu) Cofactor for various enzymes
Iodine (I) Aids in improvement of growth of roots and callus
Manganese (Mn) Cofactor for various enzymes involved in respiration and photosynthesis
Molybdenum (Mb) Cofactor for enzymes involved in converting nitrate to ammonium
Zinc (Zn) Activator of a large number of enzymes. Shortened internodes and smaller leaves are the deficiency symptoms
Organic compounds
Sugars Energy source. Sucrose is widely used as sugar and osmoticum. Glucose, fructose and sorbitol are also used in some media formulations
Vitamins Not necessary but improves the culture. Thiamine (B1) is essential for carbohydrate metabolism and some amino acids biosynthesis. Nicotinic acid (niacin) and pyridoxine (B6), biotin, folic acid, ascorbic acid (vitamin C) and tocopherol (vitamin E) are also used in some media
Myo-Inositol Sugar alcohol, important in cell division, membrane and cell wall development
Support substances
Agar Polysaccharide derived from red algae, most commonly used gelling agent
Agarose Derived from agar. It does not contain agropectin and its sulphate groups. Used in protoplast and another culture where impurities are not desired
Gellan gums Gellan gums such as Gelrite and Phytagel consist of a polysaccharide produced by bacterium Pseudomonas elodea. They are clear and thus makes detection of contamination easier than using agar
Plant Growth Regulators
Plant growth regulators (also called plant hormones) are numerous chemical substances that profoundly influence the growth and differentiation of plant cells, tissues and organs. Plant growth regulators function as chemical messengers for intercellular communication. There are currently five recognized groups of plant hormones- auxins, gibberellins, cytokinins, abscisic acid (ABA) and ethylene. Ethylene is mainly involved in abscission and flower senescence in plants and is rarely used in plant tissue culture. In addition to the five principal growth regulators brassinosteroids and polyamines are also used in plant tissue culture.
Since plant culture media is a rich source of nutrients it can also support the growth of microorganisms; consequently they are sterilized prior to use, usually by autoclaving. Heat labile culture media components are filter sterilized and later added to the media to prevent degradation of these compounds. Manipulations of culture media after autoclaving and plant material is carried out in sterile working environment provided by Laminar flow-cabinets.
Fig.1. (A) Callus formation on a tobacco leaf explant in the presence of 1 ppm of each 6-benzylaminopurine (BA) and 1- naphthaleneacetic acid (NAA), after 21 days in culture. (B) Direct bud formation on a tobacco leaf explant in the presence of 2 ppm BA and 0.5 ppm NAA after 24 days in culture. (C) Indirect bud formation from callus on a tobacco leaf explant in the presence of 2 ppm BA and 1 ppm NAA after 30 days in culture.
Types of cells, Tissues, and Organs for Explants isolation
The purpose of the culture, genotype of the plant, physiological and developmental state of the plant plays a key role in determining the cells, tissues, or organs to be used to establish the culture and the success of the culture. Shoots, meristem tips, leaves, stems, roots, flower organs, seeds, seedlings, and embryos serves as a source for isolation of explants. Shoot apical meristems are used for virus free clonal propagation, for germplasm storage (cryopreservation). Terminal and axillary buds isolated from young actively growing shoots serves as most potential explants to initiate plant cell cultures. Leaves are used as an explants source for callus production, young actively growing leaves can regenerate buds directly at the cut surface of explants in media supplemented with high cytokinins to auxins. They can also regenerate indirectly through callus. Leaves were also the first organs used to isolate protoplasts from mesophyll tissue. Stems are used as explant source for callus production from pith parenchyma cells, or bud regeneration from the vascular tissue. Root tip being the most responsive section are easy to culture. Roots are also used in liquid cultures in bioreactors for the production of secondary metabolites by transformation with Agrobacterium rhizogenes. Anthers and ovules are used for haploid plant production.
General Methodology used in Plant Tissue Culture
Plant tissue culture procedure, initiated with a small part of plant (explant) and ends up with the development of acclimatized plant is divided into following five stages.
Stage 0: Pretreatment
Stage I: Establishment
Stage II: Generation of propagules
Stage III: Preparation for external environment
Stage IV: Acclimatization in the external environment
Stage 0: Pretreatment of Mother Plant and Explant
Each and every step in plant culture is quite critical and enough attention must be paid for each step. This is the first step in development of a plant cell culture. One should be wise enough for the selection of appropriate starting material. In general the explants selected from field grown plants has a high risk of contamination issues, to reduce these starting material is collected from plants grown under controlled condition. The response of the explants can be increased by specific environmental and chemical treatments of stock plants, the plants from which explants are collected for culture.
Stage I: Establishment of Aseptic Culture
The aim of this step is to obtain an aseptic culture and initiate growth of the plant material. Choice of the starting material will depend on the technique employed and the plant species being cultured. Care should be taken to select the disease free material which is capable of active growth. Material taken from seedlings parts like hypocotyls and cotyledons are usually more responsible than the ones collected from adult plants. This step concentrates more predominantly on the preparation of explants and establishment of culture. Explants preparation is initiated by washing of the plant material collected under the running tap water with 2-3 drops of detergent like teepol. Then the material is manipulated inside the laminar flow-cabinets. Surface sterilization is the key step for initiation of contamination free culture, it is usually carried out with germicidal agents like alcohol or sodium hypochlorite or mercuric chloride singly or in combination depending up on the material employed. Then traces of the surface sterilant are removed by washing several times with sterile distilled water. Antibiotics and fungicides are used to control contaminants.
Explant preparation will lead to the induction of wounding responses, few of these may be detrimental to the continued growth of culture. Under such circumstances material may be treated with antioxidants or adsorbents to remove toxic compounds. The type of sterilizing agent and antioxidants or adsorbents employed solely depends on the material employed. As this step aims at initiation of growth of the culture this may be achieved by stress treatments like cold, osmotic and nutrient starvation. Stress treatments are common in general for the induction of embryogenesis in isolated cells, and particular for androgenesis and gynogenesis. Plant gowth hormones, viz., auxins and cytokinins play a vital role in induction of organogenesis and embryogenesis.
Stage II: Generation of Suitable Propagules
This stage aims at the development of propagules that can give rise to whole plants. These could be axillary or adventitious shoots; branched or adventitious roots; haploid, double haploid, or somatic embryos; or storage or propagative organs. Propagules may be derived via organogenesis, by shoots, roots, storage or propagative organs produced adventitiously, or by their de novo formation from callus, nodules, meristems, or suspension cultures. Alternatively, propagules may be derived via somatic embryogenesis, androgenesis, or gynogenesis, where the somatic cells are reprogrammed to form embryos.
Stage III: Preparation for External Environment
This step aims at preparing the plant material developed to survive in external environment, in order to survive in the external environment the planlet has to start photosynthesizing and survive away from artificial supply of carbohydrate. Shoots of appropriate length are rooted prior to transfer, some may form adventitious roots on shoots in culture but mostly roots are induced by the supply of specific auxins to the culture media. In contrast the shoots are regenerated on root cultures by the supply of cytokinins.
Stage IV: Acclimatization in External Environment
Plants grown in tissue culture will be exposed to high humidity and low levels of light, as a result they will be poorly developed and have limited photosynthetic capacity on exposure to external environment. The plants are gradually acclimatized to external environment after washing the agar from the roots.
Callus and cell suspension cultures
Initially peripheral cells of the wounded cut explants gets induced for cell division, later the inner cell layers under the damaged cells reaches the meristematic state by dedifferentiation resulting in increased cell division leading to callus tissue formation. Callus is made up of parenchymatic cells. 2,4- dichlorophenoxy acetic acid (2,4-D), is the most effective auxin for callus induction. Callus fragments can be transferred to liquid media in flasks on rotary shakers. Cell suspension cultures are used for the production of secondary metabolites. Cells in suspension undergo redifferentiation to proembryonic clusters by auxin, often without or with low levels of cytokinin treatment. Upon removal or lowering the auxin level the proembryonic clusters develop somatic embryos. Callus tissue in light develops chlorophyll and becomes green but remains white in dark.
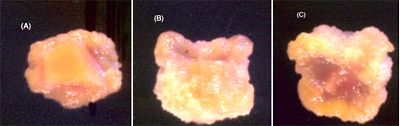 Fig.2. Stages in callus formation in a carrot root explant in the presence of 2 ppm 2,4-D and 0.5 ppm BA after (A) 10, (B) 18, and (C) 26 days in culture.
Fig.2. Stages in callus formation in a carrot root explant in the presence of 2 ppm 2,4-D and 0.5 ppm BA after (A) 10, (B) 18, and (C) 26 days in culture.
Somatic Cell Hybridization
Prtoplasts are isolated cells without cell walls. They are isolated either by mechanical means or by use of cell wall degrading enzymes principally from mesophyll tissue of leaf, callus or suspension cultures and from pollen grains. Isolation procedure initially involves plasmolysis of cells by the osmoticum, mannitol followed by cell wall degradation with the mixture of cellulase, hemicellulase and pectinnase. Protoplasts serve as a good material to understand cell wall development. Protoplast serves as a chief source for somatic cell hybridization to overcome crossing barriers and incompatibilities in breeding programs for crop improvement. Fusion of protoplasts is enabled either by positively charged medium composed of polyethylene glycol, high level of Ca2+ , and pH ranging from 5.6 to 6.6 or by alternating electric fields, termed as electro fusion.
Another important application of protoplast application is production of cybrids, where an enucleated protoplast is fused with other protoplast carrying the nuclear genetic material. Cybrids contain nuclear genetic information of only one parent.
Anther and Pollen culture
Tissue culture facilitates the production of haploid plants from the culture of microspores, uninucleated pollen cells. Totipotent pollen gametophyte can be induced to divide and form haploid somatic cells in vitro either inside the intact anther or after isolation. The haploid somatic cells can be used to produce homozygous, double haploid lines after chromosome doubling by colchine treatment. Double haploids are used for the production of isogenic lines in breeding programs of several crop plants. The use of isolated microspores serves as better system for haploid production because the dividing, diploid anther tissue cells cannot be separated from the microspore derived haploid cells. Amino acids and activated charcoal are added to the culture medium used for haploid production to enhance cell division.
Morphogenesis and Organization Aspects in Culture
The totipotent nature of almost all cells to express their genetic stability and regenerate whole plant is on one of the fundamental assets of plant tissue culture. Isolated cells and tissues undergo dedifferentiation to ground state and can be induced to redifferentiate to new cells, tisues, organs and a whole plant through manipulation of various factors in tissue culture medium. Plant regeneration can be either by organogenesis or somatic embryogenesis. The development of a unipolar structure after dedifferentiation culminates in the formation of an organ, a shoot, root leaves or flowers. Equal amount of auxins and cytokinins in the culture medium lead to callus formation. Lowering the auxin level to cytokinin results in initiation of shoot primordia where as increasing the auxin ratio to cytokinin induces root formation. Somatic embryogenesis occurs when bipolar meristem develops into proembryo and later into somatic embryo with embryonal axis consisting of both shoot and root meristems. The proembryo development follows similar pathway followed by zygotic embryos. The problem of hyperhydricity, glossy vitreous leaves lacking normal mesophyll development and distorted cuticular and stomatal structure, is more predomianat in liquid cultures than semi solid agar cultures.
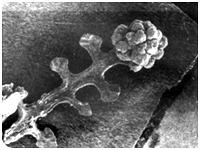 Fig.3. Meristematic bud clusters on a fern leaf cultured in liquid medium in the presence of 2 ppm KN after 24 days.
Fig.3. Meristematic bud clusters on a fern leaf cultured in liquid medium in the presence of 2 ppm KN after 24 days.
Applications of Plant Tissue culture
Micropropagation
The first and most important commercial application of the plant tissue culture. It is used for the rapid, large scale multiplication of herbaceous and woody plant species including forest species through enhanced axillary bud formation, organogenesis and or somatic embryogenesis. Existing buds in shoot tips and axillary buds makes them an obvious choice as explants to initiate the micropropagation. In other types of explants like leaves, roots, or stem tissue which do not have existing buds the regeneration involves adventitious bud formation through dedifferentiation and redifferentiation.
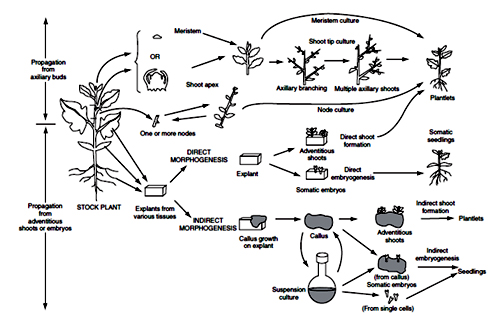 Fig.4. The strategies used to multiply plants in vitro from axillary buds and from adventitious buds. George EF (1993) Plant Propagation by Tissue Culture: Part 1 – The Technology. Basingstoke: Exegetics
Fig.4. The strategies used to multiply plants in vitro from axillary buds and from adventitious buds. George EF (1993) Plant Propagation by Tissue Culture: Part 1 – The Technology. Basingstoke: Exegetics
Secondary Metabolite Production
Plants are store houses of innumerable phytochemicals, which has immense potential in pharmaceutical, food and cosmetic industry applications. The ability of plant cells to produce secondary metabolites in in vitro culture was first established in liquid cultures. Secondary metabolite production by in vitro culture suffers a limitation of inability to produce as much as the amount produced in vivo.
Germplasm Storage
Rare, unique, endangered, and selected plant material can be conserved by tissue culture techniques. Cells, calluses, embryos, buds, shoot tips, ovules, and anthers can be stored and regrown later based on the necessity. In vitro conservation can be for short term of 8-12 monthes under growth-limiting conditions like low temperatures and by use of high osmoticum like 3-5% mannitol and growth inhibiting conditions by including inhibitors like abscisic acid, cycocel, paclobutrazol or for a long term in at ultra low temperatures in liquid nitrogen (cryopreservation). Dimethy sulfoxide, ethylene glycol or propylene glycol, sorbitol or sucrose are used as cryoprotectants. The cultures are freezed at -1960c in liquid nitrogen and stored at -80 to -1000c or in liquid nitrogen to prevent ice formation and damage to the tissue.
Plant Transformation and Genetic Manipulation in vitro, Transgenic Development
Transgenic plant development relies greatly on plant tissue culture to introduce new genes into the plants. Transgenic development is one of the most important applications of plant tissue culture which was first established in model system, tobacco and later extended to all plant genera of both dicotyledons and monocotyledons. Plant tissue culture techniques are used for the transformation and regeneration of the plants where as molecular biology techniques are employed for the development of gene constructs and for confirmation of the transgenecity of the trangenic plant, both plant tissue culture and molecular biology goes hand in hand in the development of transgenic plants.
Prerequisites for transgenic plant development are, firstly availability of a competent tissue capable of regenerating the entire plant, secondly an efficient transformation method, thirdly a suitable selection system for regenerating transformed plants with reasonable frequency. Many technological advancements have happened in all the aspects involved in the of transgenic development which lead to the development and commercialization of many transgenics plants, starting with the commercialization of flavor savor tomato, in 1996 in USA to the Bt cotton in India, in 2002. Till date, Seven transgenic crops—maize, cotton, canola, rapeseed, potato, squash, and papaya—are being produced commercially in 12 countries, including the United States, Argentina, Canada, China, and India.
References
George, E.F. (1993) Plant Propagation by Tissue Culture: Part 1 – The Technology. Basingstoke: Exegetics.
Karabi, D. and Swapan, K. D .(2007) Transformation Methods and Impact. In Encyclopedia of Plant and Crop Science. Taylor and Francis: New York, Published online: 12 Dec 2007; 1233-1237.
Sage, D.O., and Ian, J. P. (2007) Plant Cell Tissue and Organ Culture: Concepts and Methodologies. In Encyclopedia of Plant and Crop Science, Taylor and Francis: New York, Published online: 12 Dec 2007; 934-938.
Vitaly, C., Stanislav, V. K., Benoît, L., Adi, Z., Mery, D. Y., Shachi, V., Andriy, T., and Tzvi, T.(2007) Biological systems of the host cell involved in Agrobacterium infection. Cellular Microbiology. 9(1), 9–20.
The history of plant tissue culture dates back to1904, where Hannig isolated immature embryos in vitro from several brassicacae members and recovered fertile plants. Discovery of plant tissue culture medium, MS medium by US plant physiologists Murashige and Skoog in 1962 sped up the developments in the tissue culture techniques. Since then the science of plant tissue culture developed to its present state. The plant tissue culture techniques are based in part upon the ability of plants to reproduce asexually (vegetative reproduction) and completely on cell competence and totipotency, i.e. the ability of plant cells to express their genetic potential, to undergo dedifferentiation and redifferentiation, and to regenerate whole plant.
However all the plant cells do not have the ability to develop complete plant. Plant cell and tissue culture includes the techniques involved in the culture of aseptically isolated cells, protoplasts, tissues, and organs on a defined sterile medium under controlled environmental conditions. The regeneration of new plant may be either by organogenesis – where unipolar organ formation takes place or by somatic embryogenesis – where bipolar embryonic axis forms.
Plant tissue culture techniques serves as a valuable tool for studying the cell physiology and metabolism, this forms the basis for a major practical application, i.e., the production of secondary metabolites – phytochemicals for the food and pharmaceutical industries.
Propagation in plant tissue culture is used to develop high quality pathogen free plants. Micropropagation has an advantage of rapid, large scale multiplication of new genotypes and generation of pathogen free plants over conventional propagation.
The outstanding progress and remarkable achievements in plant tissue culture techniques complemented with developments in molecular biology, including embryo rescue, haploid production, somatic cells hybridization and genetic transformation facilitated the production of novel plant crops. Plant transformation relies on tissue culture methods to generate new crop plants for crop improvement. Transformation, introduction of DNA into plant cells was achieved either by biological vector like Agrobacterium or virus or by physical means like particle bombardment or chemical techniques like PEG mediated protoplast transformation. Each method has its own merits as well as demerits which should be considered along with the crop and explants to be transformed.
Regeneration of haploid cells and plants from isolated microspores enabled the production of homozygous plants for nonconventional breeding. These homozygous plants could be used to generate double haploids by doubling the chromosome number with in short time which otherwise takes a long time through conventional breeding program. Isolation and culture of protoplast has enabled us to study the plant cell wall apart from providing a system for somatic hybridization through protoplast fusion to overcome interspecific crossings barriers. Culture of young embryos allowed embryo rescue techniques used to overcome embryo abortion resulting from incompatible crossings of several species.
Isolated plant cell or organ, or tissue, used to initiate an in vitro culture is called as explant. Plant tissue culture techniques can be broadly divided into organized and unorganized cultures, based on the morphology of the established cultures. The term unorganized cultures is more general and includes all cell cultures where as organized cultures includes organ cultures in which some form of organized growth is maintained, as in the continued growth of shoot or root apices.
Organized cultures may contain mixtures of single cells and unorganized cell clumps, as well as organized structures. Similarly, unorganized cells can also be induced to organize and regenerate plant organs through organogenesis or embryogenesis.
Regeneration by organogenesis or embryogenesis can take place either directly from isolated cells, tissues, or organs or from an intermediate unorganized mass of cells called the callus/tissue phase. Direct regeneration may occur from isolated tissues or organs that regenerate shoots or roots, or from cells induced to form embryos. Indirect regeneration occurs following the proliferation of unorganized cells established in tissue (callus) or cell suspension culture, and may also occur from semiorganized callus or nodules produced on pieces of tissue or organ isolated in culture.
Factors which could have a play in plant tissue culture
Plant tissue culture techniques are very sensitive and greatly affected by internal environment of the explants as well as external environment. Success in tissue culture is greatly determined by nutrient media used for in vitro culture, conditions of in vitro culture and the plant material used as a source of explants.
Nutrient medium consists of minerals, vitamins, carbohydrates, plant growth regulators and a solidifying gelling agent in case of semisolid cultures. Each component of nutrient medium has an immense effect on the culture and proper care should be taken for selecting specific medium for specific explants and crop species.
Temperature and the amount of light and duration have either inhibiting or enhancing effect on the response of plant tissue in vitro and needed to be adapted for each plant or tissue requirements. Usually cultures are kept under low light intensities. The physiological status of the tissue has a paramount role in response of isolated cultured explants. Usually juvenile and young actively growing tissues respond better to hormonal signals than older tissues in culture medium.
Laboratory Facilities
The facilities of tissue culture laboratories are organized into three major areas, one is used for all nonaseptical chores like media preparation and autoclaving and for initial plant preparation. The second area is used for performing all the aseptical procedures of explant isolation, sterilizatrion, planting and for subculturing the cultures in a laminar air hood. The third area is used for maintenance of the cultures under controlled temperature and light quality and duration as well as humidity as specified for each plant culture.
Nutrient Medium
Success of plant tissue culture is greatly dependent on selection of the culture medium. Typical, culture media includes minerals supplied in the form of their salts for providing macro and micro nutrients, classified based on their requirement. It also includes a carbon source, usually sucrose. Apart from the essential ingredients culture media also includes vitamins and amino acids. Culture media also includes the most vital compounds called as plant growth hormones, which in very low concentrations can alter the plant growth and development (morphology).
Many specific culture media formulations are developed for different stages of culture progression or for individual species, of all the media formulations available till date one developed by Murashige and Skoog is widely used. Plant material can be cultured in a liquid medium or in a semisolid medium partially solidified with a gelling agent such as agar or gellan gum. Cultures grown in a semisolid medium are kept static, whereas liquid cultures are usually agitated to ensure adequate gaseous exchange.
Components of Nutrient medium and their role
Media component Role/Significance
Macroelements
Calcium (Ca) Cofactor for many enzymes and cell wall synthesis
Magnesium (Mg) Cofactor for enzymes, integral component of chlorophyll molecule and cation in plants to balance negative ions
Nitrogen (N) Constituent of amino acids, proteins, nucleic acid, certain hormones and chlorophyll
Phosphorus (K) Integral part of nucleic acids and other structural compounds in plants
Potassium (K) Major positive ion to balance negative ion in plants
Sulphur (S) Important in disulphide bond formation in tertiary protein structure. Constituent of the vitamins thiamine and biotin and of coenzyme A, which has a key role in fatty acid metabolism and electron transfer reactions of photosynthesis
Iron (Fe) Required for chlorophyll synthesis and in many oxidation and reduction reactions. Iron is added in chelated form as ferrous-EDTA to prevent precipitation
The Microelements
Boron (B) Essential in enzyme activity in lignin biosynthesis and metabolism of phenolic acids. Deficiency inhibits cell division and elongation in root tip and shoot tip meristems
Cobalt (Co) Not considered as essential element, may be toxic at higher concentrations
Copper (Cu) Cofactor for various enzymes
Iodine (I) Aids in improvement of growth of roots and callus
Manganese (Mn) Cofactor for various enzymes involved in respiration and photosynthesis
Molybdenum (Mb) Cofactor for enzymes involved in converting nitrate to ammonium
Zinc (Zn) Activator of a large number of enzymes. Shortened internodes and smaller leaves are the deficiency symptoms
Organic compounds
Sugars Energy source. Sucrose is widely used as sugar and osmoticum. Glucose, fructose and sorbitol are also used in some media formulations
Vitamins Not necessary but improves the culture. Thiamine (B1) is essential for carbohydrate metabolism and some amino acids biosynthesis. Nicotinic acid (niacin) and pyridoxine (B6), biotin, folic acid, ascorbic acid (vitamin C) and tocopherol (vitamin E) are also used in some media
Myo-Inositol Sugar alcohol, important in cell division, membrane and cell wall development
Support substances
Agar Polysaccharide derived from red algae, most commonly used gelling agent
Agarose Derived from agar. It does not contain agropectin and its sulphate groups. Used in protoplast and another culture where impurities are not desired
Gellan gums Gellan gums such as Gelrite and Phytagel consist of a polysaccharide produced by bacterium Pseudomonas elodea. They are clear and thus makes detection of contamination easier than using agar
Plant Growth Regulators
Plant growth regulators (also called plant hormones) are numerous chemical substances that profoundly influence the growth and differentiation of plant cells, tissues and organs. Plant growth regulators function as chemical messengers for intercellular communication. There are currently five recognized groups of plant hormones- auxins, gibberellins, cytokinins, abscisic acid (ABA) and ethylene. Ethylene is mainly involved in abscission and flower senescence in plants and is rarely used in plant tissue culture. In addition to the five principal growth regulators brassinosteroids and polyamines are also used in plant tissue culture.
Since plant culture media is a rich source of nutrients it can also support the growth of microorganisms; consequently they are sterilized prior to use, usually by autoclaving. Heat labile culture media components are filter sterilized and later added to the media to prevent degradation of these compounds. Manipulations of culture media after autoclaving and plant material is carried out in sterile working environment provided by Laminar flow-cabinets.
Fig.1. (A) Callus formation on a tobacco leaf explant in the presence of 1 ppm of each 6-benzylaminopurine (BA) and 1- naphthaleneacetic acid (NAA), after 21 days in culture. (B) Direct bud formation on a tobacco leaf explant in the presence of 2 ppm BA and 0.5 ppm NAA after 24 days in culture. (C) Indirect bud formation from callus on a tobacco leaf explant in the presence of 2 ppm BA and 1 ppm NAA after 30 days in culture.
Types of cells, Tissues, and Organs for Explants isolation
The purpose of the culture, genotype of the plant, physiological and developmental state of the plant plays a key role in determining the cells, tissues, or organs to be used to establish the culture and the success of the culture. Shoots, meristem tips, leaves, stems, roots, flower organs, seeds, seedlings, and embryos serves as a source for isolation of explants. Shoot apical meristems are used for virus free clonal propagation, for germplasm storage (cryopreservation). Terminal and axillary buds isolated from young actively growing shoots serves as most potential explants to initiate plant cell cultures. Leaves are used as an explants source for callus production, young actively growing leaves can regenerate buds directly at the cut surface of explants in media supplemented with high cytokinins to auxins. They can also regenerate indirectly through callus. Leaves were also the first organs used to isolate protoplasts from mesophyll tissue. Stems are used as explant source for callus production from pith parenchyma cells, or bud regeneration from the vascular tissue. Root tip being the most responsive section are easy to culture. Roots are also used in liquid cultures in bioreactors for the production of secondary metabolites by transformation with Agrobacterium rhizogenes. Anthers and ovules are used for haploid plant production.
General Methodology used in Plant Tissue Culture
Plant tissue culture procedure, initiated with a small part of plant (explant) and ends up with the development of acclimatized plant is divided into following five stages.
Stage 0: Pretreatment
Stage I: Establishment
Stage II: Generation of propagules
Stage III: Preparation for external environment
Stage IV: Acclimatization in the external environment
Stage 0: Pretreatment of Mother Plant and Explant
Each and every step in plant culture is quite critical and enough attention must be paid for each step. This is the first step in development of a plant cell culture. One should be wise enough for the selection of appropriate starting material. In general the explants selected from field grown plants has a high risk of contamination issues, to reduce these starting material is collected from plants grown under controlled condition. The response of the explants can be increased by specific environmental and chemical treatments of stock plants, the plants from which explants are collected for culture.
Stage I: Establishment of Aseptic Culture
The aim of this step is to obtain an aseptic culture and initiate growth of the plant material. Choice of the starting material will depend on the technique employed and the plant species being cultured. Care should be taken to select the disease free material which is capable of active growth. Material taken from seedlings parts like hypocotyls and cotyledons are usually more responsible than the ones collected from adult plants. This step concentrates more predominantly on the preparation of explants and establishment of culture. Explants preparation is initiated by washing of the plant material collected under the running tap water with 2-3 drops of detergent like teepol. Then the material is manipulated inside the laminar flow-cabinets. Surface sterilization is the key step for initiation of contamination free culture, it is usually carried out with germicidal agents like alcohol or sodium hypochlorite or mercuric chloride singly or in combination depending up on the material employed. Then traces of the surface sterilant are removed by washing several times with sterile distilled water. Antibiotics and fungicides are used to control contaminants.
Explant preparation will lead to the induction of wounding responses, few of these may be detrimental to the continued growth of culture. Under such circumstances material may be treated with antioxidants or adsorbents to remove toxic compounds. The type of sterilizing agent and antioxidants or adsorbents employed solely depends on the material employed. As this step aims at initiation of growth of the culture this may be achieved by stress treatments like cold, osmotic and nutrient starvation. Stress treatments are common in general for the induction of embryogenesis in isolated cells, and particular for androgenesis and gynogenesis. Plant gowth hormones, viz., auxins and cytokinins play a vital role in induction of organogenesis and embryogenesis.
Stage II: Generation of Suitable Propagules
This stage aims at the development of propagules that can give rise to whole plants. These could be axillary or adventitious shoots; branched or adventitious roots; haploid, double haploid, or somatic embryos; or storage or propagative organs. Propagules may be derived via organogenesis, by shoots, roots, storage or propagative organs produced adventitiously, or by their de novo formation from callus, nodules, meristems, or suspension cultures. Alternatively, propagules may be derived via somatic embryogenesis, androgenesis, or gynogenesis, where the somatic cells are reprogrammed to form embryos.
Stage III: Preparation for External Environment
This step aims at preparing the plant material developed to survive in external environment, in order to survive in the external environment the planlet has to start photosynthesizing and survive away from artificial supply of carbohydrate. Shoots of appropriate length are rooted prior to transfer, some may form adventitious roots on shoots in culture but mostly roots are induced by the supply of specific auxins to the culture media. In contrast the shoots are regenerated on root cultures by the supply of cytokinins.
Stage IV: Acclimatization in External Environment
Plants grown in tissue culture will be exposed to high humidity and low levels of light, as a result they will be poorly developed and have limited photosynthetic capacity on exposure to external environment. The plants are gradually acclimatized to external environment after washing the agar from the roots.
Callus and cell suspension cultures
Initially peripheral cells of the wounded cut explants gets induced for cell division, later the inner cell layers under the damaged cells reaches the meristematic state by dedifferentiation resulting in increased cell division leading to callus tissue formation. Callus is made up of parenchymatic cells. 2,4- dichlorophenoxy acetic acid (2,4-D), is the most effective auxin for callus induction. Callus fragments can be transferred to liquid media in flasks on rotary shakers. Cell suspension cultures are used for the production of secondary metabolites. Cells in suspension undergo redifferentiation to proembryonic clusters by auxin, often without or with low levels of cytokinin treatment. Upon removal or lowering the auxin level the proembryonic clusters develop somatic embryos. Callus tissue in light develops chlorophyll and becomes green but remains white in dark.
 Fig.2. Stages in callus formation in a carrot root explant in the presence of 2 ppm 2,4-D and 0.5 ppm BA after (A) 10, (B) 18, and (C) 26 days in culture.
Fig.2. Stages in callus formation in a carrot root explant in the presence of 2 ppm 2,4-D and 0.5 ppm BA after (A) 10, (B) 18, and (C) 26 days in culture.Somatic Cell Hybridization
Prtoplasts are isolated cells without cell walls. They are isolated either by mechanical means or by use of cell wall degrading enzymes principally from mesophyll tissue of leaf, callus or suspension cultures and from pollen grains. Isolation procedure initially involves plasmolysis of cells by the osmoticum, mannitol followed by cell wall degradation with the mixture of cellulase, hemicellulase and pectinnase. Protoplasts serve as a good material to understand cell wall development. Protoplast serves as a chief source for somatic cell hybridization to overcome crossing barriers and incompatibilities in breeding programs for crop improvement. Fusion of protoplasts is enabled either by positively charged medium composed of polyethylene glycol, high level of Ca2+ , and pH ranging from 5.6 to 6.6 or by alternating electric fields, termed as electro fusion.
Another important application of protoplast application is production of cybrids, where an enucleated protoplast is fused with other protoplast carrying the nuclear genetic material. Cybrids contain nuclear genetic information of only one parent.
Anther and Pollen culture
Tissue culture facilitates the production of haploid plants from the culture of microspores, uninucleated pollen cells. Totipotent pollen gametophyte can be induced to divide and form haploid somatic cells in vitro either inside the intact anther or after isolation. The haploid somatic cells can be used to produce homozygous, double haploid lines after chromosome doubling by colchine treatment. Double haploids are used for the production of isogenic lines in breeding programs of several crop plants. The use of isolated microspores serves as better system for haploid production because the dividing, diploid anther tissue cells cannot be separated from the microspore derived haploid cells. Amino acids and activated charcoal are added to the culture medium used for haploid production to enhance cell division.
Morphogenesis and Organization Aspects in Culture
The totipotent nature of almost all cells to express their genetic stability and regenerate whole plant is on one of the fundamental assets of plant tissue culture. Isolated cells and tissues undergo dedifferentiation to ground state and can be induced to redifferentiate to new cells, tisues, organs and a whole plant through manipulation of various factors in tissue culture medium. Plant regeneration can be either by organogenesis or somatic embryogenesis. The development of a unipolar structure after dedifferentiation culminates in the formation of an organ, a shoot, root leaves or flowers. Equal amount of auxins and cytokinins in the culture medium lead to callus formation. Lowering the auxin level to cytokinin results in initiation of shoot primordia where as increasing the auxin ratio to cytokinin induces root formation. Somatic embryogenesis occurs when bipolar meristem develops into proembryo and later into somatic embryo with embryonal axis consisting of both shoot and root meristems. The proembryo development follows similar pathway followed by zygotic embryos. The problem of hyperhydricity, glossy vitreous leaves lacking normal mesophyll development and distorted cuticular and stomatal structure, is more predomianat in liquid cultures than semi solid agar cultures.
 Fig.3. Meristematic bud clusters on a fern leaf cultured in liquid medium in the presence of 2 ppm KN after 24 days.
Fig.3. Meristematic bud clusters on a fern leaf cultured in liquid medium in the presence of 2 ppm KN after 24 days.Applications of Plant Tissue culture
Micropropagation
The first and most important commercial application of the plant tissue culture. It is used for the rapid, large scale multiplication of herbaceous and woody plant species including forest species through enhanced axillary bud formation, organogenesis and or somatic embryogenesis. Existing buds in shoot tips and axillary buds makes them an obvious choice as explants to initiate the micropropagation. In other types of explants like leaves, roots, or stem tissue which do not have existing buds the regeneration involves adventitious bud formation through dedifferentiation and redifferentiation.
 Fig.4. The strategies used to multiply plants in vitro from axillary buds and from adventitious buds. George EF (1993) Plant Propagation by Tissue Culture: Part 1 – The Technology. Basingstoke: Exegetics
Fig.4. The strategies used to multiply plants in vitro from axillary buds and from adventitious buds. George EF (1993) Plant Propagation by Tissue Culture: Part 1 – The Technology. Basingstoke: ExegeticsSecondary Metabolite Production
Plants are store houses of innumerable phytochemicals, which has immense potential in pharmaceutical, food and cosmetic industry applications. The ability of plant cells to produce secondary metabolites in in vitro culture was first established in liquid cultures. Secondary metabolite production by in vitro culture suffers a limitation of inability to produce as much as the amount produced in vivo.
Germplasm Storage
Rare, unique, endangered, and selected plant material can be conserved by tissue culture techniques. Cells, calluses, embryos, buds, shoot tips, ovules, and anthers can be stored and regrown later based on the necessity. In vitro conservation can be for short term of 8-12 monthes under growth-limiting conditions like low temperatures and by use of high osmoticum like 3-5% mannitol and growth inhibiting conditions by including inhibitors like abscisic acid, cycocel, paclobutrazol or for a long term in at ultra low temperatures in liquid nitrogen (cryopreservation). Dimethy sulfoxide, ethylene glycol or propylene glycol, sorbitol or sucrose are used as cryoprotectants. The cultures are freezed at -1960c in liquid nitrogen and stored at -80 to -1000c or in liquid nitrogen to prevent ice formation and damage to the tissue.
Plant Transformation and Genetic Manipulation in vitro, Transgenic Development
Transgenic plant development relies greatly on plant tissue culture to introduce new genes into the plants. Transgenic development is one of the most important applications of plant tissue culture which was first established in model system, tobacco and later extended to all plant genera of both dicotyledons and monocotyledons. Plant tissue culture techniques are used for the transformation and regeneration of the plants where as molecular biology techniques are employed for the development of gene constructs and for confirmation of the transgenecity of the trangenic plant, both plant tissue culture and molecular biology goes hand in hand in the development of transgenic plants.
Prerequisites for transgenic plant development are, firstly availability of a competent tissue capable of regenerating the entire plant, secondly an efficient transformation method, thirdly a suitable selection system for regenerating transformed plants with reasonable frequency. Many technological advancements have happened in all the aspects involved in the of transgenic development which lead to the development and commercialization of many transgenics plants, starting with the commercialization of flavor savor tomato, in 1996 in USA to the Bt cotton in India, in 2002. Till date, Seven transgenic crops—maize, cotton, canola, rapeseed, potato, squash, and papaya—are being produced commercially in 12 countries, including the United States, Argentina, Canada, China, and India.
References
George, E.F. (1993) Plant Propagation by Tissue Culture: Part 1 – The Technology. Basingstoke: Exegetics.
Karabi, D. and Swapan, K. D .(2007) Transformation Methods and Impact. In Encyclopedia of Plant and Crop Science. Taylor and Francis: New York, Published online: 12 Dec 2007; 1233-1237.
Sage, D.O., and Ian, J. P. (2007) Plant Cell Tissue and Organ Culture: Concepts and Methodologies. In Encyclopedia of Plant and Crop Science, Taylor and Francis: New York, Published online: 12 Dec 2007; 934-938.
Vitaly, C., Stanislav, V. K., Benoît, L., Adi, Z., Mery, D. Y., Shachi, V., Andriy, T., and Tzvi, T.(2007) Biological systems of the host cell involved in Agrobacterium infection. Cellular Microbiology. 9(1), 9–20.
Published date : 31 May 2014 05:13PM










